Analysis by Dr. Joseph Mercola
July 05, 2024
STORY AT-A-GLANCE
- A peer-reviewed study in Forensic Science International found that 73.9% of post-COVID-19 vaccination deaths were directly caused by or significantly contributed to by the injections
- The study, initially censored by The Lancet, analyzed 325 autopsy cases and found cardiovascular issues were the most common cause of death, followed by blood and respiratory problems
- Researchers suggest the spike protein in COVID-19 vaccines may be responsible for side effects, potentially causing inflammation and clotting in various tissues and organs
- Another study in South Korea found increased incidences of mild cognitive impairment and Alzheimer's disease within three months of COVID-19 vaccination, particularly with mRNA vaccines
- The article mentions censorship of research critical of COVID-19 vaccines and suggests seeking help from organizations like FLCCC for those experiencing post-vaccination injuries
A bombshell study that The Lancet1 pulled within 24 hours is finally seeing the light of day. Now published in the peer-reviewed Forensic Science International journal, the systematic review of autopsy findings in deaths after COVID-19 shots revealed 73.9% “were directly due to or significantly contributed to” by the injections.2 Canadian oncologist and cancer researcher Dr. William Makis posted on X:3
“This is a victory of SCIENCE over CENSORSHIP!! Incredible perseverance by first author Nicolas Hulscher who didn't give up after LANCET pulled our paper within 24 hours after 100,000s of downloads for no legitimate reason. Big pharma put the squeeze on @TheLancet but has failed to stop us.
Our paper was delayed by one year, and those actions of CENSORSHIP and CANCELLATION led to many deaths that could have been prevented. This paper could be a game changer.”
High Likelihood of ‘Causal Link’ Between COVID-19 Shots and Death
Researchers including Makis, cardiologist, internist and epidemiologist Dr. Peter McCullough and Nicolas Hulscher, an epidemiologist with the University of Michigan School of Public Health, searched for all published autopsy and necropsy reports relating to COVID-19 shots through May 18, 2023.
Their systematic review included 44 papers with 325 autopsy cases and one necropsy case, which is an autopsy performed on an animal. The average age of the people in these reports was 70.4 years. Most often, the cardiovascular system was involved in the death, followed by hematological issues, or blood problems, and respiratory issues. In 21 of the cases, three or more organ systems were involved.
The mean time from vaccination to death was 14.3 days, but most deaths occurred within a week of the last shot. Three doctors who are experts in figuring out causes of death and studying diseases looked at each case separately.
They carefully examined all the information available for each person who died and concluded that in 73.9% of the cases, COVID-19 shots either directly caused or played a significant role in the person's death. Among them, the primary causes of death were:4
| Sudden cardiac death (35%) | Pulmonary embolism (12.5%) |
| Myocardial infarction (12%) | Vaccine-induced immune thrombotic thrombocytopenia (VITT) (7.9%) |
| Myocarditis (7.1%) | Multisystem inflammatory syndrome (4.6%) |
| Cerebral hemorrhage (3.8%) |
“The consistency seen among cases in this review with known COVID-19 vaccine mechanisms of injury and death, coupled with autopsy confirmation by physician adjudication, suggests there is a high likelihood of a causal link between COVID-19 vaccines and death,” the researchers concluded.5
What Was The Lancet Trying to Hide? ‘Lots and Lots of Vaccine Deaths’
In the video above from The Jimmy Dore Show, they discuss why The Lancet almost immediately pulled the concerning study.6 The journal stated, “This preprint has been removed by Preprints with The Lancet because the study's conclusions are not supported by the study methodology. Preprints with The Lancet reserves the right to remove a paper that has been posted if we determine that it has violated our screening criteria.”7 As noted by The Daily Sceptic on July 6, 2023:8
“Without further detail from the Preprints with the Lancet staff who removed the paper it is hard to know what substance the claim that the conclusions are not supported by the methodology really has. A number of the authors of the paper are at the top of their fields so it is hard to imagine that the methodology of their review was really so poor that it warranted removal at initial screening rather than being subject to full critical appraisal.
It smacks instead of raw censorship of a paper that failed to toe the official line. Keep in mind that the CDC has not yet acknowledged a single death being caused by the COVID mRNA vaccines. Autopsy evidence demonstrating otherwise is clearly not what the U.S. public health establishment wants to hear.”
The censored paper has now been peer-reviewed, however, and its findings add further support to those who have been sounding the alarm about COVID-19 shot dangers all along. The researchers explain, “We found by independent adjudication that 73.9% of deaths were attributable to fatal COVID-19 vaccine injury syndromes,” adding:9
“These results corroborate known COVID-19 vaccine-induced syndromes and show significant, temporal associations between COVID-19 vaccination and death involving multiple organ systems, with a predominant implication of the cardiovascular and hematological systems.
Criteria of causality from an epidemiological perspective have been met including biological plausibility, temporal association, internal and external validity, coherence, analogy, and reproducibility with each successive case report of death after COVID-19 vaccination combined with population-based studies describing mortality among the vaccinated.
Our findings amplify concerns regarding COVID-19 vaccine adverse events and their mechanisms.”
To answer the question of why The Lancet pulled the paper so quickly, The Vigilant Fox said:10
“Another COVID ‘conspiracy theory’ becomes reality as a bombshell study CENSORED by The Lancet has now been peer-reviewed. What were they trying to hide, you ask? Lots and lots of vaccine deaths.”
What Makes COVID-19 Shots So Deadly?
Most of the COVID-19 injections are linked to deaths, which suggests they share a common factor causing side effects, most likely the spike protein, the study suggests.11
Spike proteins can circulate in your body after infection or injection, causing damage to cells, tissues and organs. “Spike protein is a deadly protein,” McCullough said.12 It may cause inflammation and clotting in any tissue in which it accumulates.13 In fact, some suggest that spike protein in COVID-19 shots was designed to cause severe disease.
In a study published in the journal Science, by researchers with the National Institutes of Health’s National Institute of Allergy and Infectious Diseases, it’s revealed that the S-2P spike protein used in several COVID-19 shots binds more strongly to the ACE2 receptors in the heart, lungs, kidneys and endothelial cells of blood vessels in the body compared to the spike protein of the original SARS-CoV-2 virus.14
“Given the average time (14.3 days) in which cases died after vaccination, a temporal association between COVID-19 vaccination and death among most cases is further supported by the finding that SARS-CoV-2 Spike mRNA vaccine sequences can circulate in the blood for at least 28 days after vaccination,” the featured study notes.15
Further, it’s been revealed that the spike protein on its own is enough to cause inflammation and damage to the vascular system, even independent of a virus.16 The spike protein is known to have deleterious effects on the heart, and COVID-19 vaccine-induced myocarditis and heart attack are well-described in peer-reviewed studies.17 Multisystem Inflammatory Syndrome (MIS) has also been reported after COVID-19 shots in both adults and children.
“A possible mechanism by which MIS occurs after vaccination could be the systemic distribution of the LNPs [lipid nanoparticles] containing mRNA after vaccine administration and the consequent systemic Spike protein expression and circulation resulting in system-wide inflammation,” the researchers explain.18 Given the study findings, they suggest that anyone who receives a COVID-19 shot should be monitored for at least one year:19
“The implications of our study apply to cases of unanticipated death without antecedent illness among COVID-19 vaccine recipients. We can infer that in such cases, death may have been caused by COVID-19 vaccination.
Further urgent investigation is required to build upon our results and further elucidate the pathophysiologic mechanisms of death with the goal of risk stratification and avoidance of death for the large numbers of individuals who have taken or will receive one or more COVID-19 vaccines in the future.
Autopsies should be performed on all diseased individuals that have received one or more COVID-19 vaccines. Clinical monitoring of COVID-19 vaccine recipients is indicated for a period of at least one year after vaccination to ensure the absence of serious adverse events that may lead to death.”
Potential Link Between COVID-19 Injections and Alzheimer’s Disease
In addition to deaths related to the cardiovascular system, McCullough and colleagues found COVID-19 shot-related deaths also involved the hematological system, pulmonary embolism and the respiratory system, while adverse events related to the gastrointestinal, immunological and neurological systems have also been reported after COVID-19 shots.20
A separate study investigated the association between COVID-19 shots and the onset of Alzheimer’s Disease and mild cognitive impairment (MCI).21 The study involved 558,017 individuals in Seoul, South Korea, who were divided into two groups — those who received a COVID-19 shot and those who did not.
The findings showed an increased incidence of MCI and Alzheimer’s in those who received a COVID-19 injection, particularly in those who received mRNA shots, within three months post-injection. The mRNA vaccine group exhibited a significantly higher incidence of Alzheimer’s compared to the unvaccinated group.
The study suggests a potential link between COVID-19 shots, particularly mRNA injections, and increased incidences of Alzheimer’s disease and MCI. “This underscores the need for further research to elucidate the relationship between vaccine-induced immune responses and neurodegenerative processes, advocating for continuous monitoring and investigation into the vaccines' long-term neurological impacts,” the researchers stated.
Rampant Censorship Downplays the Truth About COVID-19 Shot Dangers
In another example of the rampant censorship surrounding COVID-19 adverse effects, a now-retracted narrative review published in the journal Cureus called for a global moratorium on mRNA COVID-19 shots.22 The review cited significant increases in serious adverse events among those who received the injections, along with an “unacceptably high harm-to-reward ratio.”23
When factoring in absolute risk and the “number needed to vaccinate” (NNV), a metric used to quantify how many people need to be vaccinated to prevent one additional case of a specific disease, the review found “for every life saved, there were nearly 14 times more deaths caused by the modified mRNA injections.”24
As for the paper’s retraction, McCullough, one of the paper’s authors, called it a “stunning act of scientific censorship.”25 In addition to calling for a global moratorium on mRNA COVID-19 shots, the authors of the paper said the shots should be immediately removed from the childhood vaccine schedule, while boosters should also be suspended.
“It is unethical and unconscionable to administer an experimental vaccine to a child who has a near-zero risk of dying from COVID-19 but a well-established 2.2% risk of permanent heart damage based on the best prospective data available,” the paper notes.26
The moratorium is warranted based on the shots’ risks of serious adverse events, the mechanisms behind those adverse events, mortality data and issues with inefficacy, vaccine control and processing.27 As with the featured Lancet study, the Cureus study was already incredibly popular, with more than 330,000 views/reads/downloads in one month compared to the average Cureus paper, which gets only 2,700 in an entire year.28
With each unwarranted retraction, more people will begin to ask questions about why this crucial information is continuing to be censored and withheld from the public instead of openly debated and presented to the public.
Injured by a COVID-19 Shot? Here’s Help
If you’ve had a COVID-19 shot and developed any unusual symptoms, seek out help from an expert. The Front Line COVID-19 Critical Care Alliance (FLCCC) has a treatment protocol for post-jab injuries. It’s called I-RECOVER and can be downloaded from covid19criticalcare.com.29
Dr. Pierre Kory, who cofounded the FLCCC, has transitioned to treating the vaccine injured more or less exclusively. For more information, visit DrPierreKory.com. McCullough is also investigating additional post-jab treatments, which you can find on PeterMcCulloughMD.com. Finally, if you’re suffering from long vax, be sure to review my strategies for boosting mitochondrial health to allow your body to heal.
- Sources and References
- 1, 7 Preprints with The Lancet July 5, 2023 (Archived)
- 2, 4, 5, 9, 11, 15, 17, 18, 19, 20 Forensic Science International June 21, 2024
- 3 X, William Makis MD, June 21, 2024
- 6 YouTube, The Jimmy Dore Show July 26, 2023
- 8 The Daily Sceptic July 6, 2023
- 10 X, The Vigilant Fox June 21, 2024
- 12 Rumble, Dr. Peter McCullough, Therapeutic Nihilism and Untested Novel Therapies, October 5, 2021, 6:00
- 13 World Council for Health, Spike Protein Detox Guide
- 14 Science. 2020 Mar 13; 367(6483): 1260–1263
- 16 Circulation Research March 31, 2021
- 21 QJM: An International Journal of Medicine May 28, 2024
- 22 Cureus January 24, 2024
- 23 Cureus January 24, 2024, Abstract
- 24 Cureus January 24, 2024, Review
- 25, 28 Substack, Courageous Discourse February 24, 2024
- 26 World Tribune February 5, 2024
- 27 Children’s Health Defense January 29, 2024
- 29 Covid19criticalcare.com
What Are the Risks & Benefits of Each Vaccine? A Comprehensive Analysis
The COVID-19 vaccines have provided a once-in-a-lifetime opportunity to answer this important question.
vnninfluencersMay 16, 2024
This article originally appeared on The Forgotten Side of Medicine and was republished with permission.
Guest post by A Midwestern Doctor
A major problem I see throughout the scientific and political sphere is that people cannot maintain a perspective that allows them to see the whole picture; rather they tend to focus or fixate on things they have some type of emotional or subconscious priming to focus on (this has been an issue throughout history). This is why you can say have someone be around an individual they like and primarily register the one good thing the individual did (while ignoring all the bad things) and conversely why they will ignore all the good things another individual they don’t like is trying so hard to do and focus on the one bad thing that individual did.
This human tendency ends up becoming a huge problem because the media will emotionally condition the public to focus on the one side on an issue which favors its corporate sponsors. This in turn leads to these people getting up in arms about that one point when individuals who dissent against the corporate narrative try to highlight the issues that greatly outweigh any purported benefit of the narrative.
This is particularly common with complex issues (which are difficult to understand to begin with) and one of my longstanding frustrations has been that despite the harms of certain vaccines greatly outweighing their benefits, many people you bring this up with can only register the danger of the (often insignificant disease) the vaccine allegedly protects against. In my eyes, one of the upsides about COVID-19 is that this selective reframing of reality and the medias lies to maintain it went to such an extreme extent, much of the public became able to realize it was absurd and started taking the time to try and fully understand the subject rather than blindly trust the “safe and effective” narrative.
One of the common questions I get from readers relates to another complex question—which vaccines are safe for their kids, and which ones are a bad idea? This is surprisingly difficult to answer because you must weigh the likelihood of an adverse event from a vaccination vs. the likelihood of suffering a complication from the disease that the vaccine would prevent you from getting (along with how effective the vaccine is in preventing the disease) and then compute a figure that takes the weighted average of each into consideration.
In order make this determination, you need to consider all of the following:
Disease Risk
How likely is it for a person to get the disease?
Some diseases we vaccinate against are incredibly rare (e.g., tetanus).
How likely is the disease to cause a negligible, minor, moderate, severe, or fatal complication?
It is very important to distinguish between these categories because, for most infections, the risk of you catching it and then it becoming a severe condition is extremely low. For example, a Neisseria meningitidis infection (which can cause septic meningitis) is really badand can progress very quickly, but also very rare for people to develop (one in ten people are asymptomatic neisseria carriers whereas approximately one in a million get septic meningitis from it each year).
How likely is it that the severity of the disease can be improved with an existing medical treatment?
Most of the infections we vaccinate against are very easy to treat. Unfortunately, the focus is always on vaccinating against the disease rather than providing treatment for it (especially if the treatment is something more unorthodox than an antibiotic). In the case of COVID-19, while severe complications represent the minority of cases, they (and the more minor ones) can in most cases easily be prevented by early outpatient treatment. Unfortunately, the Federal government has refused to disclose to the public what the effective treatments are for it (presumably because it would make it impossible to continue making money off COVID-19).
How likely is it that you will have access to the necessary treatment before you get seriously ill?
Although I dislike the vaccine approach, I have to acknowledge that this is one of the strongest arguments for it. For rapidly progressing diseases, for those in isolated areas, for those unable to recognize their need to seek medical care, and for those of limited economic means, they often cannot get the necessary treatment for the disease before it is too late to prevent a severe complication.
In general, it’s very rare that a vaccine-preventable disease has both a significant likelihood that you will get it and a significant likelihood that it will develop into a severe condition. Many of those believed to fall into this category are no longer an issue in the United States (e.g., polio or smallpox), regardless of whether or not you are vaccinated, but people who look at this question are often fixated on the past presentations of the disease when it was more pathogenic or when we did not have a way to treat it.
Vaccine Efficacy
How likely is the vaccine to be effective in preventing the disease, and do the presence of vaccine antibodies correlate with a decreased risk of the disease?
Many vaccines fail to do one or both of these. COVID-19 has provided the greatest red pill in history on this topic, especially since successive COVID-19 vaccines actually increase your risk of catching the disease.
How likely is the vaccine to be effective at preventing severe complications of the disease?
The human papillomavirus vaccine (which “prevents” cervical cancer) is an excellent example of a vaccine that does not live up to its promise to do so because its promise was based on a series of erroneous (and wishful) assumptions.
How long does the vaccine’s protection last following immunization?
Many vaccines suffer from the problem of declining immunity, hence needing repeated boosters to be given which re-expose the recipient to the risks of the vaccine. COVID-19 again has provided the greatest red pill in history on this topic, as the immunity from it wanes approximately 3 months after the most recent injection.
How likely will it be for the vaccine to prevent you from getting the disease when you need to be protected?
The hepatitis B vaccine is routinely given at birth, and then twice more very early in life. This is nonsensical for two reasons. First, at the time of birth, infants lack an immune system that can mount a proper antibody response to the vaccine. Second, hepatitis B is spread by blood-to-blood contact (e.g., sharing heroin needles or having unprotected sex), both things very unlikely to happen in early childhood. This is important because the hepatitis B vaccine typically only lasts for around 6-7 years (estimates vary). The best explanation I have seen for why the vaccine is given immediately following birth (despite being completely unjustifiable) is that it habituates parents to come in for regular well child vaccination visits starting at two months.
How long does it take for the vaccine to create a selective pressure that causes the pathogen to no longer be covered by the vaccine?
This is a huge problem for any vaccine that “works”, because it rapidly creates selective pressure for variants not covered by the vaccine’s antigen. The only vaccines that do not suffer from this issue are the ones where the vaccine does not create selective pressures against the vaccine (e.g., against the non-contagious tetanus bacteria’s toxin) and live attenuated vaccines since they contain so many different antigens [note: except for tuberculosis, all live attenuated vaccines are viruses]. Live attenuated vaccines, unfortunately, can cause infections of the vaccine strain in the immunocompromised host, and are frequently contaminated with other viruses that were present in the medium used to cultivate the virus.
Because this is a longstanding problem, many theorized that the COVID-19 vaccine (due to it only containing a single antigen in a rapidly mutating part of the spike protein) would rapidly trigger the production of pathogenic variants. This is, of course, what happened soon after it hit the market.
Does the vaccine have other benefits besides preventing the disease?
Some live attenuated vaccines broadly stimulate the immune system. In third world countries with a high infectious disease burden, this actually saves lives (this has been shown with the measles-mumps-rubella vaccine [MMR] and the tuberculosis vaccine [BCG]) because the immune system is better able to fight off otherwise fatal infections modern medical care is not available for.
Note: conversely, other vaccines like DPT, when studied were found to do the opposite and broadly increase the risk of death due to the immune suppression they create.
Population Immunity
Assuming that the vaccine “works”:
Does vaccination creating a selective pressure for vaccine resistant variants to produce more or less dangerous variants?
With certain vaccines, the strains created by the selective pressure of the vaccine are more dangerous than those that preceded them, and they affect different age groups. This has primarily been shown with the childhood vaccines for bacterial infections.
Does developing a population-wide vaccine immunity to a disease improve or worsen the disease’s consequences?
Two of the best examples of this were the chickenpox vaccine and the measles vaccine (two relatively benign diseases in the era preceding vaccination due to a robust herd immunity).
If you get chickenpox as a child, it is benign, but if you get it as an adult, it can often give you a horrible (and sometimes recurrent) case of shingles. The CDC eagerly expected rolling out the chickenpox vaccine would decrease shingles, but the opposite instead happened (so they, of course, suppressed the data). The researcher who conducted those studies, with a good basis for doing so, theorized that this happened because the reduction of chickenpox in the population prevented people from having their immune response to it be periodically boosted by natural exposure.
In the case of measles, if there is no pre-existing immunity and poor living conditions (e.g., widespread vitamin A deficiency), the disease can be horrible (e.g. measles killed 10% of Native Americans it infected in one outbreak). In the past, infants received antibodies from their mother’s milk (the importance of breast feeding is discussed here), which provided them sufficient protection to build up permanent natural immunity once they were exposed to the virus. The population-wide herd immunity we used to have does not exist now, and periodic measles outbreaks still occur despite the majority of the population being vaccinated. Because we lack that immunity, many are vulnerable to measles, which is always addressed by vaccinating even more people for the disease.
Is there a benefit to developing the disease naturally that is prevented by vaccination?
One of the lesser known facts about diseases is that childhood infections are often critical for helping the immune system develop. A variety of diseases that are much more severe in adults than their corresponding “vaccine preventable” childhood infections are observed to result from not catching the disease in childhood. Some examples include:
-Not having a chickenpox infection increasing your risk of glioblastoma (a horrible brain cancer) later in life.
-Not having a mumps infection increasing your risk of ovarian cancer (one of the most deadly cancers for women).
Note: this research substantiating these links and more can be found here.
To further appreciate the consequences of disrupting the existing microbial ecosystem with vaccinations, I would highly recommend watching this video. What essentially happened after the bacterial childhood vaccinations were introduced, was that it caused both that infection and other bacterial infections to become more common and for them to mutate to more dangerous strains that affected many people who were not previously susceptible to them and created a variety of new side effects from the infections not seen before. The response to the other infections becoming worse has been to make new vaccines for them, which has further accelerated this downward spiral.
Vaccinating While Infected
If you are already infected at the time you receive the vaccine, does this improve or worsen your response to the infection?
This was a major problem with the human papillomavirus (HPV) vaccine, as it was shown in the study data that Merck submitted to the FDA that if you had a pre-existing HPV-16 or -18 infections, your risk of developing a cancerous lesion was increased by 44.6% following vaccination. I also have now seen many things which suggest that getting a vaccine while you are infected with COVID-19 significantly worsens the infection.
If an existing infection worsens following vaccination, how practical is it to test for the infection prior to vaccination, and vaccinate at a later time?
As far as I know, a pre-existing infection is never tested for before vaccination. I presume that this is because public health authorities never want to do anything which might encourage vaccine hesitancy. This is particularly absurd with COVID-19 because we are continually passing out free tests and encouraging people to test multiple times per week…except when they are going to be vaccinated.
Note: I have come across numerous cases of individuals with an active (but relatively inconsequential) COVID infection who received a vaccine and then became severe ill from their pre-existing infection.
Vaccine Side Effects
How likely is the vaccine to cause a minor, moderate, severe, or fatal side effect?
One of the important things to understand about toxins is that their side effects distribute on a bell curve, which means that their side effects become increasingly rarer as they increase in severity. Although the severe reactions are the most noticeable (e.g., the rapid progression to lifelong autism or sudden infant death syndrome), less severe chronic complications are much more common, and in my opinion, create the greatest burden to society (this is very well illustrated by Edward Dowd’s figures below).
An explosion of chronic illness (particularly of neurological and autoimmune nature) in our society has paralleled the mass vaccination of society. This has been most apparent at three times in history: the period of the smallpox vaccines, after 1986 when Congress passed legislation to shield manufacturers from liability for producing dangerous vaccines (which led to a rapid increase in the number of childhood vaccinations and no motivation to ensure their safety), and following the COVID-19 vaccines. In each case, we’ve tragically become acclimated to an increase in baseline levels of chronic illness which never existed in the past, and we have simply assumed that the current disease burden is normal, when in reality it is not.
Similarly, although the sudden deaths from the COVID-19 vaccine are tragic, many less severe but debilitating or disabling reactions are much more common.
How easy is it to recognize that these effects occurred?
Given how difficult it is to get doctors to acknowledge the most extreme reactions to a childhood vaccine, it should come as no surprise that the more subtle issues go mostly unrecognized or are dismissed (to the point that members of the societal orthodoxy commonly produce memes making fun of anti-vaxxers who blame their various health issues on vaccines).
One of the struggles I have experienced throughout my career in medicine is the fact that I can notice right away that a vaccine injury has occurred while sadly, most of my peers cannot. Most of the signs that scream out to me are rarely detected by my colleagues, and the symptoms either don’t register or they give some type of innocuous explanation for them (e.g., it’s a behavioral thing that requires an SSRI to treat—something I do not support). Furthermore, if I try to point them out, all it accomplishes is undermining my credibility.
This has been particularly fascinating to watch with COVID-19, as countless patients are all developing the same symptoms after vaccination, and yet most doctors ardently insist they have nothing to do with the vaccines. Fortunately, this does appear to be beginning to change, as the medical field’s eyes are opening up to the issue (in part because many healthcare workers have also been injured).
How consistent and safe is the vaccine’s manufacturing process?
Because vaccine manufacturers are exempt from liability for unsafe products they produce, many corners often end up getting cut with the production process so more money can be made by the manufacturer (to this point America’s facilities that make our vaccines have been plagued with production concerns such as potential contamination) the FDA has done almost nothing to address. Additionally, since many vaccines are grown in cell cultures, contamination from things already present in the cells (e.g., retroviruses) is inevitable, and some believe this is a key issue with the vaccines.
With the COVID-19 vaccines, it has been demonstrated that much less due diligence was done with producing the vaccines (likely due to Operation Warp Speed enabling this malfeasance) and as a result, there is immense variation in what is present in each vaccine. Presently, this is the best explanation I have found for why people react so differently to the vaccines and why “hot lots” exist.
Does the vaccine priming your immune system to target one pathogen reduce its ability to respond to other pathogens or cancerous cells within the body?
This is a frequent but under-appreciated consequence of vaccination. As far as I know, the worst offender in this regard has been the COVID-19 vaccines, which have been linked to both an explosion of cancers and unusual diseases typically only seen in immune-suppressed individuals.
Does the vaccine impair circulation and cause microstrokes in the body?
I believe that this is one of the primary mechanisms of harm done by vaccines, and frequently what must be focused on when treating these patients (e.g., we have seen miraculous results for individuals with COVID-19 vaccine injuries who we treated with simple methods for addressing their zeta potential). As this is a complex but critically important subject to understand, I put together an article explaining it here, and a series explaining how it affects the body and how to treat it here.
Does the vaccine cause the immune system to attack the body and give rise to chronic illnesses?
All vaccines work by provoking the immune system to go into overdrive to attack the vaccine antigen that is present. The downside to this is that it typically also causes the immune system to attack other proteins in the vicinity (e.g., a mice study showed that mice develop allergies to pollen that is in the air at the time of their vaccination). Autoimmunity is especially likely to happen if the vaccine shares antigen sequences with human tissue (homologies) and contains a very strong adjuvant (the vaccine component which stimulates the immune system). Before the COVID-19 vaccines (which have a remarkable number of homologies with human tissue), Gardasil (the HPV vaccine) was the greatest offender here as it had to use a very strong adjuvant and had homologies to human tissue.
If a vaccine causes negative reactions, does the risk increase if multiple vaccines are given concurrently?
Everything I have seen has shown that the more vaccines that are given (especially if they are received at the same time), the more likely people are to develop a severe reaction to the vaccine. This, for example, is why Sudden Infant Death Syndrome has been correlated to receiving multiple vaccines simultaneously, why many parents have observed their child developing autism after multiple vaccinations, and why some doctors advocate for not following the CDC schedule and spacing out the required vaccinations.
Similarly, if the same vaccine is provided multiple times (especially if it has tissue homology) each successive time it is given, it is more likely to create an autoimmune condition. Although I have seen this with other vaccines, this effect has been by far the most dramatic with the COVID-19 vaccines because their risk of a severe adverse event increases significantly with each successive vaccination.
Although increased autoimmune priming likely plays a role, the best model I have to explain the cumulative toxicity with vaccines is largely due to them successively impairing the zeta potential of the body, which creates catastrophic consequences once a critical threshold is passed. Analogously, I often see the worst responses to vaccines in individuals who already have an impaired zeta potential and cannot tolerate the additional reduction created by one more vaccine.
Unfortunately, since vaccines are considered “safe and effective” their potential harms are never considered. This is why individuals who try to propose very simple measures that could greatly mitigate the harm of the vaccination schedule (like spacing out vaccines) are relentlessly attacked under the justification that “they are not following CDC guidelines” and thus creating vaccine hesitancy.
At this point, we have never had a study performed on the cumulative effects of children receiving the entire vaccine schedule. Anyone who tries to do so is attacked for unethically experimenting on children, since the placebo group (who are not vaccinated) are placed at a “great and unjustified” risk because they are being denied life-saving vaccines (for diseases they will never get).
Since these studies have thus far never been completed, a variety of less controlled ones (e.g., comparing vaccinated and unvaccinated children in the same medical practice) are published. While these studies show a massive number of complications arise from vaccination, they are typically dismissed as not being valid since they weren’t a controlled study, and in many cases, the authors are attacked (e.g., consider what happened to Paul Thomas). Similarly, I and many colleagues can often immediately recognize children who were never vaccinated (as they are healthier in the body, mind and spirit), yet the changes vaccination create have become so normalized in our society, most doctors now lack the ability to recognize the currently accepted baseline is not normal.
If the vaccines cause negative reactions, who is the most susceptible to them?
There is a huge variation in responses to vaccines. Typically, individuals who have had a bad reaction to a vaccine are more likely to have bad reactions in the future, and there are a variety of other signs that predict the likelihood of a bad reaction to vaccines (e.g., previous adverse reactions, pre-existing autoimmune conditions, poor physiologic zeta potential, genetic metabolic defects, having previously had the infection the vaccine is for).
Unfortunately, since vaccines are considered 100% safe, virtually nothing qualifies as an exemption to them (which California has used as a justification to revoke the licenses of anyone who writes exemptions, hence leading to it now being almost impossible to get vaccine exemptions there). To highlight the absurdity of it, I had a friend who had a documented anaphylactic reaction to the Moderna vaccine they need to go to ER for, and was simply told that they needed to get a different COVID vaccine. I have also heard of a case where someone hospitalized in a California ICU for a vaccine reactions and could not find a doctor in the state who was willing to write a medical exemption for their employer.
The Public Health Perspective
One of the largest issues with public health is that it does not see people as individuals, and instead uses theoretical constructs (that are often wrong) and applies them to the entire population. I believe that this is done because it is the most practical way for a centralized bureaucracy to affect the health of a large swath of people with whom it has no direct contact with.
This approach is a huge problem because many individuals behave differently from others (e.g., some derive no benefit from the intervention and some react poorly to the interventions). Unfortunately, for the centralized public health approach to work, the public’s diversity must be ignored, and dissent must be forcefully suppressed when members of the public complain.
Many issues in life I believe ultimately come down to people being lazy and taking the easy way out when addressing a complex problem. For example, in the recent series on SSRI antidepressants (this article and this article), one way the entire debacle could be summarized is that patients with mental health issues require a therapeutic relationship with a counselor who can help them navigate their issues, but this is far too time consuming for most doctors in practice.
Psychiatric medications offer an easy way out; you can just give the drug for the symptom, feel like you solved it, and not have to deal with the patient. Unfortunately, this often doesn’t work, and the medications make the patients worse. At this point, the choice to do one’s job properly or default to a lazy approach again comes up. The doctor can actively monitor the patient for adverse reactions to their drug and intervene before those effects are catastrophic, or gaslight the patient, tell the patient the drug works and just give them more of it or another drug. Most of the catastrophic events I’ve heard about from SSRI-injured patients happened because the doctors took the lazy approach to handle their issues.
Similarly with public health, if a contagious disease is present that the system believes needs to be addressed, there are two options:
- Adopt comprehensive public health measures that contain and mitigate the spread of the disease and encourage practices that increase the natural immunity of the population.
- Add a vaccine for it to the vaccine schedule and mandate it so everyone takes it.
Since the second approach takes much less work, it’s a foregone conclusion that it will happen. Similarly, since the approach will inevitably fail to prevent many people from catching the disease, excuses will be made for why this happens that ultimately boils down to “not enough vaccines were given.”
Likewise, it’s inevitable that injuries will occur from these campaigns (which often outweigh any benefit achieved by the vaccines). When this happens, those injuries are written off by the centralized public health administrators as “necessary collateral damage” for the greater good that the vaccine creates and system-wide policies will be adopted to conceal those injuries and gaslight the injured.
Typically, once it becomes clear that the vaccine is not completely “safe and effective” the justification provided to the public is that the vaccines create “herd immunity” to the disease, and that this benefit outweighs the negative consequences of the vaccine. Unfortunately, in most cases (for many of the reasons listed above) the vaccines do not create herd immunity and instead become a product the population needs to take indefinitely while the disease continues to persist.
Note: for those interested in this subject, I discussed how vaccines consistently fail to prevent disease transmission here, and how we watched this unfold with the COVID-19 vaccines here.
Which Vaccines Should Be Avoided?
For each vaccine, as we consider the risk of its disease, the efficacy of the vaccine, the effects of developing vaccine immunity within a population, the issues with vaccinating while infected, and vaccine side effects, it should become clear that this is an immensely complex question to answer. There are so many potential risks and benefits of different magnitudes that combining them into a weighted average borders on the impossible.
This helps to illustrate some of the major issues that arise when you provide an intervention with known harms as a preventative for a potential risk that may or may not happen (note: the same can also be said for statins). My own belief is that if a therapy has known harm, the benefit for it needs to be concrete (e.g., all antibiotics are to some extent toxic, but most would agree that toxicity is outweighed if someone has a dangerous infection the antibiotic will treat). In the case of vaccination, there are a few vaccines that can be given therapeutically (BcG, rabies, and ones made from the patient’s own serum) to treat an existing issue, so a clear understanding can be reached about the relative risks and benefits of each, but that is not the case for virtually every other vaccine on the market.
Typically speaking, to analyze complex questions like this, we depend on large clinical trials. The problem with such trials is that since they are industry-funded, they always omit most of the adverse events that arise (e.g., they reclassify a severe event as something nebulous, they use a toxic placebo to mask the increase in adverse events seen amongst the vaccinated, or they only monitor subjects for a brief period of time, which is not long enough for most of the vaccine side effects to appear). Generally speaking, the only way to get around this issue is to assess the total number of people who die in each group (as there is no way to reclassify death), and when this metric is looked at in the trials for the worst vaccines (e.g., Gardasil or Pfizer’s COVID-19 vaccine) the total death rate is shown to be increased by vaccination.
The other option is to look at population statistics. Sadly, while these consistently show vaccines cause significant harm, public health officials tend to ignore this data.
When I approach this question I use the following algorithm, where each item takes precedence over the ones after it.
- Does the vaccine have an unusually high degree of toxicity?2. Does the vaccine potentially provide an important benefit?3. Does the vaccine have other reasons to make me concerned about its potential side effects?4. Does the vaccine actually work?5. Does the vaccine still work?
I will now briefly discuss some of the vaccines on the current CDC schedule that I feel are the worst offenders.
Gardasil
First, let’s consider the HPV vaccine and the benefits it created by “preventing cervical cancer.”

While I have seen datasets (when stratified by age) showing Gardasil (and other HPV vaccines) actually increased the cervical cancer death rate in those vaccinated, I will give it the benefit of the doubt here. As the graph shows, cervical cancer rates were already approaching 0 before Gardasil, so it is difficult to say if any of the lives saved were due to it (at this point I believe the cancer prevention attributed to Gardasil is false).
Note: many other diseases whose decline was attributed to vaccination also actually had most of their decline occur prior to a vaccine being available.
However, assuming all lives were saved by Gardasil, in England, each year it has saved 6 lives per 100,000 (0.0006%) people, and in the United States, 2 lives per 100,000 (0.0006%) people. Conversely in the clinical trials, 133 per 100,000 (0.13%) participants died (in comparison, the average death rate at the time for those the same age as the trial participants was 43.7 per 100,000). This means, in the best case scenario for the vaccine, for 100,000 people you traded killing 89.3 of vaccine recipients in return for saving 2.
Even though this is terrible, the greater issue is that in the original HPV clinical trial, between 2.3% to 49% of the individuals who received Gardasil developed a new autoimmune condition. We do not know exactly where in that range the total number of new autoimmune disorders was, as Merck classified many autoimmune disorders simply as “new medical conditions” (industry trials always reclassify something they don’t want to show up in the final trial with vague labels like this), but other investigations have concluded the 2.3% figure significantly underestimated the rate of new autoimmune conditions.
So, in return for saving 2 lives per 100,000 people while killing 89.3, you are also giving 2300 (and likely many more) a new life-altering autoimmune condition. All in all, I would not say this represents the best risk-to-benefit ratio. Unfortunately, because Gardasil is so profitable, nothing has been done about this despite numerous red flags being set off and many petitions being made to the FDA to address it.
Diphtheria, Pertussis and Tetanus (DPT)
I am not a fan of the DPT vaccine for the following reasons:
- It is the vaccine most clearly linked to infant deaths (I summarized the extensive degree of evidence substantiating the link that has accumulated over the last century here).
- The vaccine frequently causes permanent brain damage (especially the older version of it). In addition to hearing this from many parents, this happened to two members of my extended family who received the slightly older and more toxic version of it.
- I believe it is one of the primary causes of childhood ear infections (one of the most common complaints parents see their pediatricians for). Many doctors have observed this link, and the best example I heard of came from a doctor and medical missionary who decided to vaccinate an ashram (Indian temple) he was staying in. Before the vaccines, ear infections were non-existent, immediately afterward a large number of children came down with them.
Conversely, I believe the benefit is minimal because:
- The vaccine does not prevent the colonization of any of these bacteria. This is why pertussis outbreaks occur in fully vaccinated populations.
- Diphtheria is now non-existent in the United States, so there is no reason to vaccinate against it (additionally it can be treated with modern antibiotics).
- Tetanus is now very rare (there are approximately 30 cases a year) and it’s actually difficult to say how much the vaccine antibodies protect a person from tetanus (studies have shownthat the vaccine produced antitoxin does not prevent tetanus).
Note: I’ve had multiple family members who went to the ER for a laceration, were told they needed to get a tetanus vaccine, agreed to on the condition it only had tetanus, but not diphtheria or pertussis, and when I reviewed their medical records, they had received the DPT vaccine.
Hepatitis B
As stated above, I do not believe childhood hepatitis B vaccines can be justified. Additionally, the vaccine does create complications and has been repeatedly associated with neuromuscular autoimmune conditions. I believe that this is most likely due to the fact that the antigen used shares a homology with myelin (the coating of nerves), but it may be for other reasons as well.
In adults who are at risk of a hepatitis B infection (e.g., healthcare workers who can accidentally get poked with infected needles), there is a much stronger justification for this practice. However, even in this case, I believe it should be the healthcare workers decision rather than a mandate since the risk vs. benefit of this vaccination has not been clearly established.
Measles, Mumps, Rubella (MMR)
As discussed above, it is a bit of a debate if the MMR vaccine decreases measles rates, since while regular vaccination does reduce measles rates, permanent immunity to it disappears within the population, and outbreaks will still occur within the vaccinated population. Sadder still, deaths from measles had almost completely disappeared at the time the vaccine for it was introduced (so there was essentially no justification for introducing it), and in effect by creating the vaccine we turned a non-existent problem into a permanent one by doing so. From my perspective, the greatest problem with the MMR vaccine is its frequent association with autism, something I believe is much worse than developing measles and something you are at a much higher risk for than the infection itself.
Polio
Two types of polio vaccines exist. The inactivated polio vaccine (currently used in the USA) and the live attenuated one (frequently used in poorer nations). The inactivated one does not prevent you from catching polio, but does to some extent (I don’t know how to calculate the exact figure) prevent a polio infection from causing polio-like paralysis. Since it does not prevent infection, it has no effect on transmission. The live polio vaccine does prevent you from becoming infected with polio, but has the unfortunate side effect of sometimes causing polio in the recipient and spreading the weakened polio virus into the environment.
At this point, the polio virus is mostly extinct, and from 2017 onwards, more cases of polio have resulted from the vaccine than polio itself (note: one of my friend’s relatives developed polio from the vaccine). One of the most tragic examples occurred in India where Bill Gates diverted their health budget to aggressively vaccinating against polio, which resulted in 491,000 children developing a “polio-like” illness.
Given that there is no reason to vaccinate against polio, there is no benefit to outweigh the vaccine’s risks. The risk from this vaccine is harder to quantify as I have met many people who have had bad reactions to it, but they did not have a consistent pattern to the injuries (which I often see with other vaccines).
Influenza
There is presently no evidence that the (often mandated) influenza vaccine prevents an individual from getting the flu (which, in most cases, is a relatively benign infection) or transmitting it to others. Additionally, there is evidence that the vaccine increases your likelihood of developing a severe case of influenza and developing influenza in the subsequent year. Furthermore, many individuals have developed injuries from the influenza vaccine.
Meningococcal
Initially, due to the severity of a Neisseria meningitidis infection, I initially thought the meningococcal vaccine would probably be a vaccine you could make a strong case for. Unfortunately, there are multiple dangerous strains of this bacteria, and one of those strains (strain B) is very difficult to make a vaccine for, since it has homology with tissue of the human body.
Not surprisingly, this has created a selective pressure on the bacteria and now the majority of infections are caused by strain B, which until recently, the scheduled vaccine did not cover (and at this point I am unsure how effective this newer vaccine is). Furthermore, as discussed above, many people carry this bacteria and are asymptomatic—the infection is very rare and the primary group at risk are those with pre-existing susceptibilities, not the general population. Additionally, tonsillectomies (an unnecessary procedure) significantly increase one’s risk for meningitis.
Conversely, the vaccine has a variety of potential autoimmune complications. By far the most common one I encounter is that it causes Crohn’s disease (typically a few months after vaccination), and I think this side effect alone outweighs any potential benefits from the vaccine.
For those wishing to learn more about this subject, I would suggest reading this article on why vaccines consistently fail to create herd immunity, Miller’s Review of Critical Vaccine Studies (especially in regards to the HiB and Pneumococcal vaccines), and the textbookVaccines and Autoimmunity. Peter Gøtzsche (one of my heroes) has also written a good review of the evidence surrounding the vaccines, Vaccines: Truth, Lies, and Controversy, which highlights many issues with them but also has the typical pro-vaccine bias and contains certain conclusions I do not agree with (but makes it an excellent book for opening the eyes of more conventional physicians). Finally, Turtles All The Way Down also does a deeper dive on many of these vaccines.
Pneumococcal and Haemophilus influenzae type B (HiB)
These two are probably the most difficult routine vaccines to have a clear-cut position on. This is because:
- These two infections, especially HiB are the vaccine-preventable illnesses that are the most likely to cause severe complications in children. For example, when the HiB vaccine came out, pediatricians around the country noticed a significant decline in the rates of infants with meningitis, which is a big deal. Similarly, in modern-day pediatrics, many of the most common concerning infections doctors encounter are pneumococcal.
- Although these vaccines have adverse effects, they are not as dangerous as those of many other vaccines.
- Because these vaccines work but target an easily mutatable part of the bacteria, their adoption triggers their target bacteria to mutate, become resistant to the vaccines, and, in some cases, affect different populations. For example, the pneumococcal vaccine is continually being updated and re-released, with additional strains being covered in each successive version (and I’ve seen multiple vaccinated children with potentially life-threatening pneumococcal infections who had been vaccinated). In the case of the HiB vaccine, it selected for the A strain (HiA), which in some areas was more deadly than HiB, and also selected for strains that affected adults (typically HiB only affects children), leading to severe HiB infections becoming a disease of adults and the elderly.For those wishing to learn more about this topic, this talkand this talk(which as previous linked to above) provide the best case I have seen against the HiB vaccine, and illustrates the issues with our oversimplification of how the human body interacts with its microbiome.
Note: many of the studies supporting the contentions in this section can be found within this excellent book.
The Risks and Benefits of the COVID Vaccines
Although many tragic things have happened with the COVID-19 vaccines, the circumstances around them have also made it possible to shed light on the actual risks and benefits of a vaccine, a topic that is typically far too obfuscated for anyone to make sense of. The clarity this time around is primarily because:
- The novel vaccines were rapidly rolled out onto the entire population at the start of 2021. This makes it possible to compare numerous existing yearly trends to before and after the deployment of the COVID vaccines.
- A lot of people strongly objected to how the vaccines were pushed onto the population, and did a lot of work to prove that the risks from these vaccines greatly outweighed their benefits in almost every aspect that was examined.
For example, many people are aware of this dataset:
Recently two things became available, which I believe help to clearly illustrate the poor risk-to-benefit ratio of the COVID-19 vaccines.
Rasmussen Reports
The first was a recent poll from Rasmussen Reports. Before discussing it, I would like to share the results from two of their prior polls on this issue:

 There are a few important takeaways from these polls:
There are a few important takeaways from these polls:
- Although Democrats tend to believe that the COVID virus is dangerous and that vaccines are safe relative to Republicans, they have now seen so much evidence to the contrary that the gap between them is much smaller. This is especially true for the vaccine deaths, which will likely have immense political repercussions for the party that forced them on America.[
- In the public’s perception, the same number of people have died from COVID-19 as from the vaccines. Given that many of the COVID-19 deaths occurred before the vaccines, many of those deaths were not actually due to COVID-19, and that the vaccines do not offer complete protection against COVID-19, this is a strong argument that the benefits of the vaccines do not outweigh their risks, especially when you factor in their much more common complications which disable but do not kill the recipient.
- Many respondents likely did not understand what “household” meant (and likely instead interpreted it to just mean someone they knew). This is because nowhere near 11% of US households have had a COVID-19 or vaccine death in them.
Note: Many people disparage Rasmussen and claim they have a right-wing bias. I, however, consider them to be one of the most accurate political polling firms in the country.
Edward Dowd
Edward Dowd has taken an innovative approach to red-pilling the public—showing the financial costs of the vaccine program for the country and making people feel like chumps for investing in fields that are being adversely affected by those costs. Since everyone can relate to money, this makes the concept much easier for individuals to grasp, and more importantly, since money is the most important thing to the upper class, they are likely to be motivated to act against the vaccination program in order to protect their assets.
Dowd has assembled a team of experienced analysts that has done a lot of work to calculate the costs of the vaccine program. Recently they released a report which speaks for itself:
When I reviewed Dowd’s report, I realized that there were a lot of issues that I know have human and economic costs that it was not counting, presumably since they are impossible to calculate. This means he had to underestimate the harms that have been caused by the vaccine program.
Because things like this are so difficult to estimate, you have to err on the conservative side and avoid claiming things you cannot quantify or are unsure of. Similarly, I have the same experience each time I write an article here, and do not mention a lot of things I am passionate about after I realize I can’t actually back them up.
Conclusion
These recent publications (and the datasets that Dowd’s estimate is based upon) show clearly and unambiguously that the risks of the COVID vaccines greatly outweigh any possible benefit they might have. Given that much of the country is beginning to see this now, it will be very interesting to see how this issue unfolds in the coming years as our institutions struggle to rebuild the trust they spent decades creating in America. My hope is that this process will allow us to also critically examine the entire vaccine program, which has by and large enjoyed complete immunity to scrutiny, due to both the difficulty in comprehensively assessing it and our institutions’s adamant protection of them.
One of the themes of my articles here has been to discuss the progressively evolving pleas for COVID amnesty, which in the space of slightly under a year have gone from “the experts were wrong, but you should still trust them rather than your gut” to “America’s COVID-19 response was based on lies.” Recently, the author of one plea (I did not completely agree with) posted something I felt made an excellent conclusion to this article.
At this point, I believe that all vaccines can cause harm frequently enough that the harm must always be considered when evaluating the vaccine. For this reason, I always feel very torn on what to do when people ask me to provide them with a way to protect themselves from the harms of a vaccine they have to get (note: the two best approaches I know of are taking a lot of vitamin C beforehand, and doing whatever you can to strengthen your zeta potential).
This is because regardless of what you do, you will still always have patients who are harmed by taking the vaccine, and I hate being complicit in what happens. To this point, I have had times where I repeatedly warned a patient against vaccinating where I felt they were at risk of an adverse reaction, and they had one anyway, and then they suffered a permanent complication and I was left having to try to help them get better.
I also believe that natural immunity is always superior to vaccine immunity. For this reason, I believe that the correct approach to handling almost all diseases you can vaccinate against is to accept the inherent risk of getting it as an unvaccinated individual and be familiar with what treatment protocol you need to implement if you got the infection so that you can clear the infection and develop natural immunity. Just imagine how different the world would be now if we had followed that approach instead of suppressing every single treatment for COVID-19 and mandating a deadly and ineffective vaccination on the population.
To learn how other readers have benefitted from this publication and the community it has created, their feedback can be viewed here. Additionally, an index of all the articles published in the Forgotten Side of Medicine can be viewed here.
This is asymmetric information. So, we have governments and media continuing to pretend a massive health crisis with chronic illness, deaths and disabilities is not going on. The data would suggest otherwise...
The data we have made public is free, but some people want projections and decision-making ideas. These are things we might end up starting a business from. I would have never thought we could. This is what asymmetric information does, and the government and the media are suppressing this information.”
A quick look at the overall casualties from the CV19 vax reveal an unparalleled medical disaster. Dowd explains:
“I went before Senator Ron Johnson in February to talk about the ‘pandemic scorecard,’ which is abysmal.
Ever since the CV19 vaccine came on, we have had 1.1 million Americans die excessively, 4 million permanently disabled and another 28 million injured. It’s 33 million people who have been negatively affected now.
The question you have to ask is why are these institutions not screaming from the rooftops? I think the reason why is, it’s all because of the (deadly) vaccine. It’s all circular, and I think it’s a joke at this point.”
Is the worst over?
The short answer from Dowd is No.
Dowd contends, “Let’s just look at the disability data..."
" We surged to a new high in June of 2023. We have not gone to a new high since. It kind of backfilled a little bit, but the last two months we have seen back-to-back increases. This is a called a plateauing effect.
If it was all clear, I would like to see that number come down. Unfortunately, it’s not.
It can start to go back down, or it can have another consolidation and another spurt upward.
The bad news is it is plateauing at a new high level.
The good news is it has not gone up to a new level, but if it does, we have problems.”
One big problem Dowd has spotted is an explosion of cancers and, yes, you cannot get the truth about this either. Dowd says:
“The fact that people will not even say that cancers are on the rise is pretty comical to me. Doctors were reporting it anecdotally, and now we have the data to prove it. This is where we are...
In 2022, I said that ‘60,000 millennials died excessively between March of 2021 and February of 2022. That was a Vietnam War.’ That tweet went viral, and Reuters and AP fact checked me and said no, our experts say that’s not true.
Now, even the establishment is saying there is excessive all-cause mortality. So, we are now in a stage where cancers are not rising. They are now denying that. The lies are just unreal.”
There is much more in the 36-minute interview.
Join Greg Hunter of USAWatchdog.com as he goes One-on-One with money manager and investment expert Ed Dowd, author of the recently updated book called “Cause Unknown: The Epidemic of Sudden Deaths in 2021, 2022 and 2023”...
You can order Dowd’s newly updated book called “Cause Unknown” by clicking here. If you want to go to Dowd’s website called PhinanceTechnologies.com, click here. Dowd’s work on compiling data on deaths and disabilities caused by the CV19 bioweapon/vax is all free at his website.
The 10 Stages of mRNA Denial
From ‘the cure’ to poison in just three years.
This article originally appeared on The Dossier and was republished with permission.
Guest post by Jordan Schachtel
Let’s take a trip down memory lane, all the way back to the ancient times of early 2021, to see how “the science” on mRNA Covid-19 shots has evolved in such a short period of time.
1) It’s the cure!
In the words of former CDC Director Rochelle Walensky: “Vaccinated people do not carry the virus and don’t get sick.”
2) It’s not the cure but it prevents you from getting it and spreading it to others
In the words of The Science himself, the vaccine prevents Covid-19 from mutating. Hooray science!
3) It doesn’t prevent you from getting it but it prevents you from getting sick
Okay, so in the UPDATED words of The Science himself, “the situation is so clear, the data affirm, if you get the vaccinated you are protected … we know that as a fact.”
4) It doesn’t prevent you from getting sick but it prevents you from getting REALLY sick
Ok ok, so it might not work all the time but it’s rare anyway!
*The “breakthrough” era commences…*

5) It doesn’t prevent you from getting REALLY sick but it prevents you from dying
Uh oh, all of those Pharma press releases and “studies” might need to undergo additional observation…
All Major COVID Vaccines in the World Were Once Declared 100% Effective, but None Actually Worked
6) It doesn’t prevent you from dying but it prevents most people from dying
So the good news is that it probably won’t harm your kids, maybe, possibly??

7) Hey, it’s still a net benefit! Trust the institutions!
With their reputations at stake, credentialed legacy science institutions continue declare the shots safe, effective, and beneficial. Have faith!

8) Okay, well at least it doesn’t actively harm people! Roll up your sleeve for the updated shot
Don’t believe those lying academics and doctors who are raising awareness about the myocarditis stuff. Take the next shot, for $cience!

9) Okay, well at least it doesn’t harm THAT many people! Sorry, I’m, uh, busy, and not rolling up my sleeve anymore
Capitulation rapidly ensues…

10) Okay, it’s poison, and it doesn’t actually do anything beneficial whatsoever. There are no benefits, only costs. The cure is so much worse than the disease. We got bamboozled every step of the way
As Pfizer and Moderna’s brands and market caps continue to hemorrhage value, more and more realize they’ve been scammed on an unbelievable level.
Study of Nearly 100 Million COVID-19 Vaccine Recipients Reveals a Host of Adverse Events
Cases of a blood clot condition called CVST were found to be three times higher than expected among the vaccinated.
By Naveen Athrappully
2/19/2024
A multinational study of over 99 million vaccinated people has identified higher incidences of neurological, cardiovascular, and blood disorder complications than what the researchers expected.
The peer-reviewed observational cohort study, published in the Vaccine journal on Feb. 12, aimed to evaluate the risk of 13 adverse events of special interest (AESI) following COVID-19 vaccination. The AESIs spanned three categories—neurological, hematologic (blood), and cardiovascular.
It reviewed data collected from more than 99 million vaccinated people from eight nations—Argentina, Australia, Canada, Denmark, Finland, France, New Zealand, and Scotland—looking at risks up to 42 days after getting the shots.
The study looked at three vaccines—Pfizer and Moderna’s mRNA vaccines as well as AstraZeneca’s viral vector jab.
Researchers found higher than expected cases that they deemed met the threshold to be potential safety signals for multiple AESIs, including for Guillain-Barre syndrome (GBS), cerebral venous sinus thrombosis (CVST), myocarditis, and pericarditis. A safety signal refers to information that could suggest a potential risk or harm that may be associated with a medical product.
GBS is a disorder in which a body’s immune system attacks the nerves, and can eventually paralyze the whole body. Most people with the condition require hospitalization. A “statistically significant increase” in GBS cases was observed after the first AstraZeneca shot. The researchers had expected 76 GBS events in the observational cohort study but ended up identifying 190.
Acute disseminated encephalomyelitis (ADEM) is a condition that typically occurs after a bacterial or viral infection. It causes inflammation of the central nervous system. Two cases were expected. However, the study identified seven events after the first Moderna jab.
Bell’s palsy is a weakness or paralysis of facial muscles. Higher than expected Bell’s palsy cases were identified after the first dose of the Pfizer and Moderna vaccines.
CVST is a condition in which blood clots form in the brain, blocking the blood from draining out. This can end up causing a hemorrhage. While 21 events were expected, researchers identified over three times the number of cases at 69 following the first dose of AstraZeneca vaccine. CVST cases were also higher than expected after the first and second Pfizer shots.
Myocarditis is inflammation of the heart muscle. Higher than expected cases of myocarditis that met the threshold for “prioritized safety signals” for the condition were “consistently identified following a first, second, and third dose of mRNA vaccines,” both Pfizer and Moderna, according to the study.
Pericarditis is an inflammation of the outer lining of the heart. The number of pericarditis cases exceeded expectations following “all doses of all the three vaccines,” researchers wrote.
Commentary From Researchers
The higher risk of GBS from vector-based vaccines like AstraZeneca has been identified in other studies as well, the researchers pointed out. Interestingly, studies on mRNA vaccines “have not observed increases of GBS,” they noted.
The researchers said that multiple other studies have also identified “increased incidence of CVST after vaccination,” which has led to the withdrawal of AstraZeneca vaccine from COVID-19 vaccine programs in several nations. Some countries have imposed age-based restrictions for the shot, they added.
Regarding myocarditis and pericarditis, researchers noted that the World Health Organization (WHO) has issued guidance on the conditions. A 2021 guidance from the WHO stated at the time that more countries were “reporting myocarditis and pericarditis in individuals who received COVID-19 mRNA vaccines.”
The Feb. 12 study noted that the U.S. Centers for Disease Control and Prevention (CDC) is monitoring and reviewing data on myocarditis and pericarditis among COVID-19 vaccinated individuals.
The study was funded by the Centers for Disease Control and Prevention (CDC), Public Health Ontario, ICES which is funded by the Ontario Ministry of Health, as well as a Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine. Researchers declared several potential competing interests.
The Epoch Times reached out to Moderna, Pfizer, and AstraZeneca for comment regarding the study.
Recent Studies Imply Vaccine Dangers
Numerous studies have shown that COVID-19 vaccines come with a risk of multiple medical complications. A recent Feb. 15 study conducted in Nordic nations concluded that booster vaccination against COVID-19 is linked to a higher risk of heart inflammation among adolescents.
The study noted that the association of myocarditis with COVID-19 mRNA vaccines has appeared “strongest in male adolescents and younger males and after the second dose.”
A separate Jan. 27 study points out that a “considerable body of evidence” suggests a correlation and even a causation between mRNA boosters and adverse effects on the immune system.
“Given the decreased severity of the virus … there are legitimate concerns about the frequent administration of boosters in immunocompromised patients, raising questions about whether this practice may be causing more harm than benefit,” it said.
A study accepted into the American Journal of Obstetrics and Gynecology on Jan. 24 (pdf) investigated placenta samples from two pregnant women who had received Pfizer and Moderna vaccines. Researchers concluded that “vaccine mRNA is not localized to the injection site and can spread systemically to the placenta and umbilical cord blood.”
Commenting on the study, cardiologist Peter McCullough said that the observations “have confirmed one of our worst fears, that is, poorly advised vaccination during pregnancy allows circulating mRNA and local production of Spike protein in the placenta potentially threatening the gestation and delivery.”
“But worse, mRNA is passed on to the baby with unknown effects on organogenesis, tissue damage, blood clotting, and a host of other adverse processes within the newborn’s body.”
A peer-reviewed article from last month which analyzed reports from the initial phase 3 trials of Pfizer and Moderna COVID-19 vaccines concluded that the shots killed more people than they saved.
Based on “conservative assumptions,“ the estimated harms of the COVID-19 mRNA vaccines ”greatly outweigh the rewards,“ the article stated, noting that ”for every life saved, there were nearly 14 times more deaths caused by the modified mRNA injections.”
Lately, evidence has been mounting suggesting the “shedding” of COVID-19 vaccine proteins. The controversial subject was largely discounted by scientists but now some doctors say that authorities are well aware of the phenomenon.
99 million patient records and they concluded that the benefits outweigh the risks!?!? We respectfully disagree.
A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals concludes that the benefits of COVID outweigh the risks. My colleagues and I disagree.
Executive summary
A new study of over 99 million vaccinated people has been highly promoted in the press with headlines like “Covid Vaccines Linked To Small Increase In Heart And Brain Disorders, Study Finds—But Risk From Infection Is Far Higher.”
I’m going to convince you that this is bullshit.
You can also read this excellent take-down by James Lyons-Weiller of the 99M patient study. The tl;dr is that the study is untrustworthy.
Results at a glance
A safe vaccine would be indistinguishable from a placebo. Does this look safe to you?
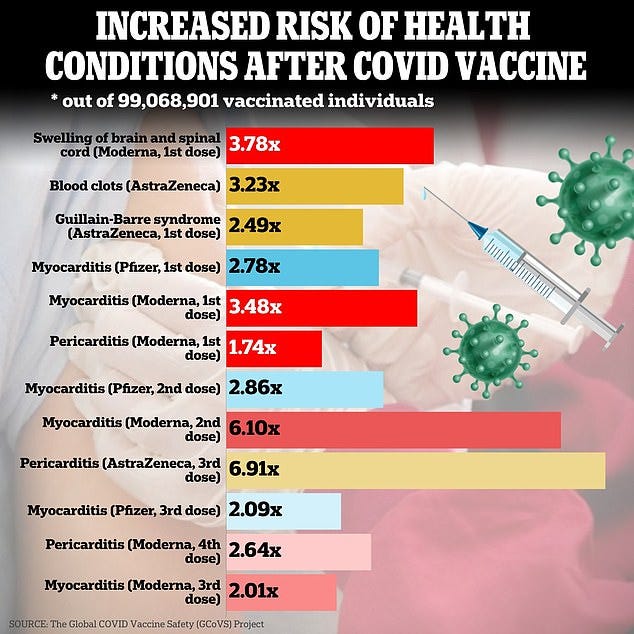
Quick summary of what the article would like you to believe
Here are the key points of the study which was funded by the CDC and HHS so no conflict of interest at all:
- They looked at the medical records of 99,068,901 vaccinated individuals.
- They compared observed vs. expected (“OE”) rates of “13 selected adverse events of special interest (AESI) across neurological, haematological, and cardiac outcomes.”
- They only looked for 42 days after the shots since everyone knows you can’t get adverse events after 42 days (I’m being sarcastic).
- They didn’t evaluate mortality due to the shot since everyone knows the vaccines are safe and didn’t kill anyone (I’m being sarcastic).
- They found clearly increased risk of the various AESI, but the end conclusion is that the risks after COVID infection are far higher, so people should take the shots. This is unbelievable. I don’t know a single cardiologist whose business dropped after the COVID vaccines rolled out. Do you?
- As usual, they aren’t allowed to share the data so you have to take their word for it.
The benefits of the COVID vaccine
There aren’t any I’m aware of.
A JAMA paper inadvertently revealed that the flu vaccine and COVID vaccine don’t work at all.
Nobody has been able to explain away that result. Nobody. Ever. They just don’t want to talk about it.
Why take a vaccine if the benefit is not measurable? That VA study was the perfect proving ground. The signal should have been large. There was no signal for either the COVID or flu vaccine. It was a stunning paper.
I always love it when a paper inadvertently blows the whole narrative out of the water and the medical community is completely clueless as to its significance.
The risks of the vaccine
The paper selected just 13 AESIs to evaluate.
But did you know that the CDC has determined that there are over 500 AESIs that were more serious than myocarditis? They simply forgot to tell people that!
Here are the actual numbers from this excellent article by Josh Guetzkow:
After dropping the new COVID-era AEs, there are 503 AEs with PRRs larger than myocarditis (PRR=3.09) and 552 with PRRs larger than pericarditis (PRR=2.82).5 This means that 66.4% of the AEs had a bigger safety signal than myocarditis and 77.3% were larger than pericarditis. You can see what those were by using this Excel file provided by the CDC and sorting the 18+ tab by the 12/14-07/29 PRR column (Column E). Then just look at which AEs have PRRs larger than the ones for pericarditis and myocarditis.
I have a question.
At a minimum, they should have warned people about myocarditis and everything more serious than that. So you should have been handed a long list of serious adverse events before you got the shot.
If you got the shot, please let me know if they warned you:
They claim that risks for GBS, etc. are higher from the virus than from the vaccine
The paper claims that even though the risks were elevated, that multiple studies show the virus itself is more dangerous:
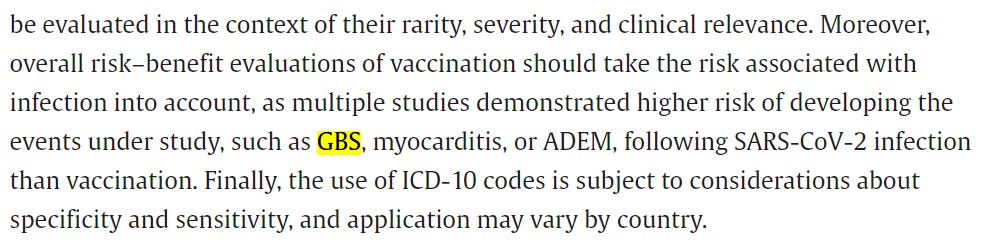
But even if that was true (which it isn’t), it doesn’t matter because, as I mentioned earlier, the vaccine doesn’t prevent you from getting COVID, so the vaccine is simply adding to the risk.
The reality that I see shows that the vaccine is way more dangerous than the virus ever was
I have huge difficulty believing that the virus is more dangerous than the vaccine. If that were true and the vaccine resulted in a net benefit, we’d be seeing cases of myocarditis DROP after the vaccines rolled out.
Where is the evidence for that?
I can’t find a single cardiologist whose myocarditis rates in kids went DOWN after the vaccines rolled out. Can you?
In a survey of 1,000 randomly selected Americans that was done by a professional market research firm, nearly half the deaths reported in the survey were ascribed to the vaccine, not to the virus. That’s a very serious safety signal. How could this study have missed it? Of course, no health authority is willing to run their own survey. They don’t really want to learn the truth.
Here’s what people actually observed in real-life, but these are just anecdotes of course:

As you can see, there is a huge disconnect here. Someone is lying to you. Big time.
Unfortunately, no pro-vax person will try to replicate this survey. They just don’t want to know for some reason.
The myocarditis risk is all over the place in the peer-reviewed literature
According to the peer-reviewed literature (Patone), myocarditis risk is elevated by 1.5 to 1.7X after a Pfizer shot, but if you got the virus, your risk of myocarditis is 11X higher if you are unvaxxed and 5X higher if you are vaxxed. So the claim in the literature is that the virus is more dangerous which is clearly at odds with the surveys above.
That same paper admitted if you are male <40, your risk of myocarditis is over 6X higher than after the virus for the 2nd dose of the Moderna shot. So if you are a male <40, vaccination makes no sense, especially since it’s all downside. But the CDC will never tell you that and the paper doesn’t make any distinction at all for different ages.
Let’s look at a paper authored by scientists at the New Zealand Ministry of Health entitled “Adverse Events Following the BNT162b2 mRNA COVID-19 Vaccine (Pfizer-BioNTech) in Aotearoa New Zealand.” They found that the COVID vaccines are indistinguishable from placebo (no statistically significant results in 11 of the 12 AESIs studied), except that there was a safety signal for myo/pericarditis which was 25.6X higher than expected in the 5-19 age group after Dose 2.
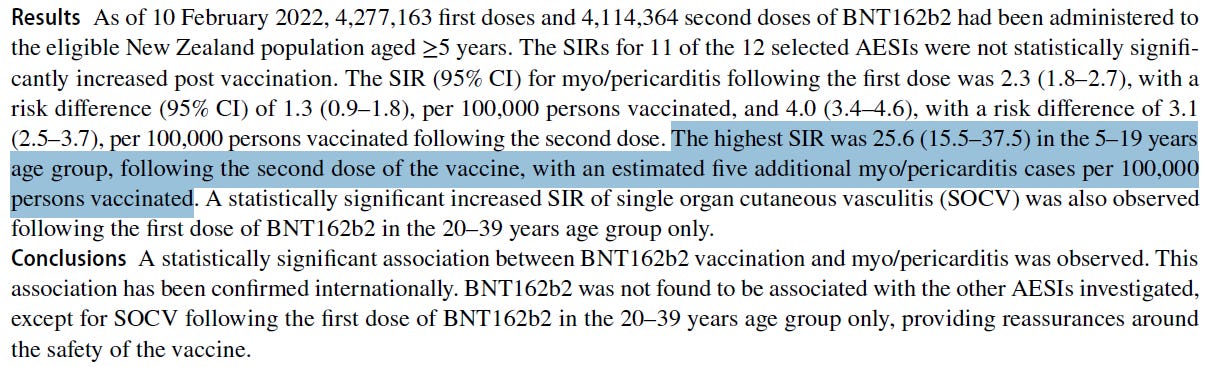
A 25.6X higher rate of myocarditis in kids is an insane safety signal. What does our CDC do about it? They recommend everyone get the shot. And top universities like Harvard require the COVID shots if you want to attend classes there as a student (faculty and staff are exempted because that’s how “science” works).
Here are the rates of myocarditis from the literature.
- Faksova (99 million study): 10 per M doses (vaccine); 40 per M infections (virus)
- Patone study: Up to 35 excess events per M doses which was same as virus on unvaccinated person; claims 23 cases per M infections for virus on vaccinated person.
- Walton (NZ study): Up to an additional 50 cases per M doses caused by the vaccine (a 25.6X increase over expected for 5-19 year olds)
- Mansanguan(Thailand): 1 in 301 13-18 year olds had confirmed myocarditis post vaccine. But more important was “Cardiovascular manifestations were found in 29.24% of patients, ranging from tachycardia or palpitation to myopericarditis.” So now we are at 3,322 cases per M doses which is 66X higher than the NZ study.
- Mueller (Switzerland): 22 out of 777 had myocardial injury after the booster shot in this study by Professor Christian Mueller in Switzerland. That’s a 2.8% rate of myocardial injury for the general population which is 800X higher than that observed from passive measurement systems.
Bottom line: Nearly 3 in 100 people experience measurable cardiac injury after the shots.
A cardiologist who I talked to who keeps a low-profile and wants to keep it that way told me, “There were no increase in cases during COVID. We started seeing an uptick in April-May 2021.” He estimated a myocarditis rate of 1 in 5,000 in 15-17 year olds whereas before the vaccine it would be unmeasurable (less than 1 in 100,000). So that’s a 20X increase which is less than what the Walton study found.
In short, the risks >> non-existent benefits.
It doesn’t add up
How can there be 770 adverse events that are generating CDC safety signals and these studies are claiming that the vaccines are safe?
The New Zealand study is stunning… they only found myo/pericarditis was elevated by 25.6X; the other 11 events studied were insignificant.
They can’t both be right because they are saying different things.
Yet from Josh’s article:
- The CDC analysis shows that the number of serious adverse events reported in less than two years for mRNA COVID-19 vaccines is 5.5 times larger than all serious reports for vaccines given to adults in the US since 2009 (~73,000 vs. ~13,000).
It’s a federal crime to make a false VAERS report. There is no benefit to the reporter to make a false VAERS report. And it’s not like the CDC did any kind of VAERS awareness campaign either.
So if the vaccine is as safe as a placebo, how do they explain all the AEs?
Summary
Read the paper. Decide for yourself who is telling you the truth.
And remember: the data used in the study is NOT available to the public.
All my attempts at data transparency have been rejected. The health authorities have determined that you get better public health outcomes if you keep the public in the dark. So all my calls for data transparency fall on deaf ears. The best “ground truth” data we have is from the New Zealand data leak…and it clearly shows that the vaccine increases mortality. The critics acknowledge I’m right, but say that “it must be due to a confounder.” More on that coming up tomorrow.
A summary of the evidence against the COVID vaccines
Here is a short list of reasons that everyone should be concerned about the COVID vaccine. This is not an exhaustive list.
- Doctors are told to trust the FDA and CDC, but not verify, when prescribing vaccines. All the post-marketing safety data is kept hidden by health authorities so not even doctors can look at the data themselves to find out if any vaccine is safe. Doctors have to trust the authorities. They are essentially told: “trust, do not verify.”
- The CDC itself doesn’t have the data to make a post-marketing independent vaccine safety assessment and they are not interested in obtaining the data either! The CDC relies on the FDA who relies on the manufacturer to test the product. The CDC could ask states for vaccination records tied to death records, but they don’t want to even ask because if they did an analysis, it could be discovered in a FOIA request. The CDC basically has no interest whatsoever in verifying what the actual safety data is.
- Lack of transparency by health authorities. Not a single health authority anywhere in the world has ever released anonymized record-level patient data for independent researchers to assess the safety of any vaccine. There isn’t any paper in a peer-reviewed journal showing that health outcomes are improved if public health data is kept secret.
- Lack of interest in data transparency by the medical community. Can you name a single high-profile pro-vaccine member of the medical community who has called for data transparency of public health data? Time-series cohort analyses can be easily produced by health authorities and published for everyone to see. These would show safety signals and do not jeopardize patient privacy. These are all kept hidden.
- We aren’t allowed to see even the simplest of charts. Wouldn’t it be great to define two cohorts on July 1, 2021: COVID vaccinated vs. COVID unvaccinated. Then you simply record the deaths from that point forward and plot them. Why isn’t this being published?
- Misinformation is deemed to be a problem, but the people making these statements are unwilling to take any steps to stop the so-called misinformation. These steps include: open public discussion to resolve differences of opinion and making public health data available/public in a way that preserves privacy. For example, HHS (as well as every state health department) should welcome all of us with open arms and invite us to query their databases (such as VSD and Medicare in the case of HHS) and publish whatever we find. Why does this information need to be hidden? The numbers tell the story, not the individual records.
- No response from health authorities to reasonable requests. I’ve sent emails to Sarah Caul of the UK ONS on four ways the ONS can increase data transparency. There was no response.
- No response when asked to explain damaging evidence. When credible scientists receive government data that shows very troubling safety signals, there is a total unwillingness of any health authority to discuss the matter and resolve it.
- The US Medicare data clearly shows mortality increases after people take the jab. Is there any epidemiologist who can explain why deaths rose during a period in time when they should have been falling (per the Medicare death data)?
- For the first 120 days after the shots given in March 2021, death rates overall were falling. But if you got the vaccine, your death rates went up. We know from data from other vaccines that the baseline death rate of 81-year olds in Medicare is 3.85%, so the baseline death rate of this group is <800 deaths a day. These deaths climb far above baseline after you took the COVID shot.
- The patient-level data released from NZ data confirms that mortality increases after the shots are given despite the fact that most of the shots were given during time periods when deaths were falling
- NZ data: Doses 2 and 4 were given while background mortality was falling, dose 3 while rising. So we’d expect the slope to fall in the first 6 months after vaccination. It does the opposite.
- Anecdotes such as the one from Jay Bonnar who lost 15 of his DIRECT friends unexpectedly since the shots rolled out. Four of the 15 died on the same day as that vaccine was given. Before the shots rolled out, Jay had lost only one friend unexpectedly. The probability this happened by chance is given by poisson.sf(14, .25) which is 6e-22. So this can’t happen by chance. SOMETHING killed Jay’s friends and 4 of the 15 died on the same day as they were vaccinated. Is there a more plausible explanation for what killed Jay’s friends? All of them who died were vaccinated with the COVID vaccines.
- Well done studies like the one done by Denis Rancourt showing 1 death per 800 shots on average. Jay Bonnar estimates he has around 14,000 friends so Jay’s numbers are consistent with Rancourt’s results.
- Survey data like Skidmore and Rasmussen Reports showing that hundreds of thousands of Americans have been killed by the COVID shots. There have never been any counter surveys published showing this not to be the case.
- The lack of any success stories. It appears that “vaccine success stories” where COVID infection fatality ratios dropped or that myocarditis cases plummeted do not exist. The US Nursing home data shows that the infection fatality rate (IFR) increased after the vaccine rolled out. There is nobody using that data making the claim it reduced the IFR.
- Anecdotes from healthcare are extremely troubling. One nurse reported a hospital admission rate that was 3X higher than anything in the 33-year history of the hospital after the COVID vaccines rolled out. Symptoms rarely ever seen were common after vaccines rolled out in that age group.
- Lack of autopsies in clinical trials and post-marketing. The CDC doesn’t request anyone to do autopsies even for people who die on the same day as they got the vaccine. Don’t they want to know what killed those people… just to be sure?
- Young people dying in sleep. There are way too many cases of young people who die in their sleep after being vaccinated. Doctors say this is a rare event. Now it is much more common. If the shots are safe, why is this happening?
- I have direct personal experience with the vaccine: two people I know were killed by the vaccine, none from COVID. I know many people who are vaccine injured from the COVID vaccine.
- Ed Dowd’s book statistics. This very popular book (“Cause Unknown”) listed 500 who died unexpectedly. Ed didn’t know how many were unvaccinated. Only one person has come forward saying that one of the people in the book who died after the vaccines rolled out was unvaccinated.
- Prominent doctor/scientists switching sides. Paul Marik is one of the top intensivists in the world. After seeing many COVID vaccine injured patients, he changed his mind about the safety of vaccines. When he was not allowed to practice medicine consistent with his Hippocratic Oath, he resigned his position.
- The corruption with COVID protocols. The COVID hospital protocols likely caused 90% of the COVID deaths in hospitals. This led to Paul Marik resigning. See details in this article. Why are doctors forced to use hospital protocols that kill a huge percentage of patients instead of using their best judgment to save patients?
- This JAMA paper shows that COVID and influenza vaccines don’t work. Why are we pushing a vaccine where the statistics clearly show the vaccines don’t work?
- The consistency of the data. There have been no counter-anecdotes showing the vaccines are safe. I keep looking for one and come up empty.
- No debates with anyone prominent promoting the government narrative. Those who promote the narrative refuse to engage in any scientific discussions to resolve differences of opinion. This is similar to the question of whether vaccines cause autism: nobody who thinks it doesn’t is willing to engage in a public discussion about it to discuss the evidence. Why not resolve the issue through dialog? It isn’t resolved in the peer-review literature where half the papers say vaccines cause autism and the other half don’t. Why can’t we talk about it?
- Fear and intimidation tactics are used to silence dissent. Open debate would be more productive. But people are not allowed to hold or discuss views that go against the “consensus” or they will lose their jobs, their certifications, or their medical licenses. Health care workers are told they will be fired if they report an adverse event to VAERS, there are nurses who won’t talk about anaphylaxis after getting the vaccine for fear of being fired, vaccine injuries are covered up, hospital workers are afraid to talk about it at work.
- The cognitive dissonance is very disturbing. When healthcare workers bring up the topic of mortality and morbidity due to the vaccine, their peers say nothing and walk away.
- Censorship tactics employed by the US government to silence dissent instead of public recorded open debates. History has shown that purveyors of censorship are always on the wrong side of the issue.
https://t.me/LauraAbolichannel/53331
A summary of the evidence against the COVID vaccines
Here's a quick summary of the key pieces of evidence that taken together show that the COVID vaccines are unsafe and that the medical community should not be trusted.

Here is a short list of reasons that everyone should be concerned about the COVID vaccine. This is not an exhaustive list.
- Doctors are told to trust the FDA and CDC when prescribing vaccines. All the post-marketing safety data is kept hidden by health authorities so not even doctors can look at the data themselves to find out if any vaccine is safe. Doctors thus have no choice but to trust the authorities since the data is kept secret. They are essentially told: “do what we tell you to do, do not question authority or we will take away your license.”

- The CDC itself doesn’t have the data to make a post-marketing independent vaccine safety assessment and they are not interested in obtaining the data either! The CDC relies on the FDA who relies on the manufacturer to test the product. The CDC could ask states for vaccination records tied to death records, but they don’t want to even ask because if they did a safety analysis, it could be discovered in a FOIA request. The CDC basically has no interest whatsoever in verifying what the actual safety data is. When I offered to show them the NZ data before I published it (so they would finally have record level data), they declined to look at it.
- Lack of transparency by health authorities. Not a single health authority anywhere in the world has ever released anonymized record-level patient data for independent researchers to assess the safety of any vaccine. There isn’t any paper in a peer-reviewed journal showing that health outcomes are improved if public health data is kept secret.
- Lack of interest in data transparency by the medical community. Can you name a single high-profile pro-vaccine member of the medical community who has called for data transparency of public health data? Time-series cohort analyses can be easily produced by health authorities and published for everyone to see. These would show safety signals and do not jeopardize patient privacy. These are always kept hidden. The lone exception is the UK ONS, but they made their “buckets” so large that you cannot see the impact of the vaccine. When I asked them to redo their analysis with smaller buckets, they stopped responding to me.
- We aren’t allowed to see even the simplest of charts. Wouldn’t it be great to define two cohorts on July 1, 2021: COVID vaccinated vs. COVID unvaccinated. Then you simply record the deaths from that point forward and plot them. Why isn’t this being published?
- Misinformation is deemed to be a problem, but the people making these statements are unwilling to take any steps to stop the so-called misinformation. These steps include: open public discussion to resolve differences of opinion and making public health data available/public in a way that preserves privacy. For example, HHS (as well as every state health department) should welcome all of us with open arms and invite us to query their databases (such as VSD and Medicare in the case of HHS) and publish whatever we find. Why does this information need to be hidden? The numbers tell the story, not the individual records.
- No response from health authorities to reasonable requests. I’ve sent emails to Sarah Caul of the UK ONS on four ways the ONS can increase data transparency. There was no response.
- No response when asked to explain damaging evidence. When credible scientists receive government data that shows very troubling safety signals, there is a total unwillingness of any health authority to discuss the matter and resolve it.
- The US Medicare data clearly shows mortality increases after people take the jab. Is there any epidemiologist who can explain why deaths rose during a period in time when they should have been falling (per the Medicare death data)?
For the first 120 days after the shots given in March 2021, death rates overall were falling. But death rates went up for those who got the shot. We know from data from other vaccines that the baseline death rate of 81-year-olds in Medicare is 3.85%, so the baseline death rate of this group is <800 deaths a day. These deaths climb far above baseline after you took the COVID shot. - The patient-level data released from NZ data confirms that mortality increases after the shots are given despite the fact that most of the shots were given during time periods when deaths were falling. Nobody’s been able to explain that.

NZ data: Doses 2 and 4 were given while background mortality was falling, dose 3 while rising. So we’d expect the slope to fall in the first 6 months after vaccination. It does the opposite. - Anecdotes such as the one from Jay Bonnar who lost 15 of his DIRECT friends unexpectedly since the shots rolled out. Four of the 15 died on the same day as that vaccine was given. Before the shots rolled out, Jay had lost only one friend unexpectedly. The probability this happened by chance is given by poisson.sf(14, .25) which is 5.6e-22. So this can’t happen by chance. SOMETHING killed Jay’s friends and 4 of the 15 died on the same day as they were vaccinated. Is there a more plausible explanation for what killed Jay’s friends? All of them who died were vaccinated with the COVID vaccines.
- Well done studies like the one done by Denis Rancourt showing 1 death per 800 shots on average. Jay Bonnar estimates he has around 14,000 friends so Jay Bonnar’s numbers are consistent with Rancourt’s results.
- Survey data like Skidmore and Rasmussen Reports showing that hundreds of thousands of Americans have been killed by the COVID shots. There have never been any counter surveys published showing this not to be the case. The Rasmussen polls have shown that a comparable number of people have been killed by the shots as by the virus (and the treatment protocols for the virus).
- The lack of any success stories. It appears that “vaccine success stories” where COVID infection fatality ratios dropped or that myocarditis cases plummeted after the vaccines rolled out do not exist. The US Nursing home data shows that the infection fatality rate (IFR) increased after the vaccine rolled out. There is nobody using that data making the claim it reduced the IFR. At best, the vaccines did absolutely nothing. If you showed someone a graph of cases and deaths, nobody would be able to tell you when the vaccines rolled out. Conversely, after the shots rolled out, the “failure stories” skyrocketed.
- Anecdotes from healthcare are extremely troubling. One nurse reported a hospital admission rate that was 3X higher than anything in the 33-year history of the hospital after the COVID vaccines rolled out. Symptoms rarely ever seen were common after vaccines rolled out in that age group.
- Lack of autopsies in clinical trials and post-marketing. The CDC doesn’t request anyone to do autopsies even for people who die on the same day as they got the vaccine. Don’t they want to know what killed those people… just to be sure?
- Young people dying in sleep. There are way too many cases of young people who die in their sleep after being vaccinated. Doctors say this is a rare event. Now it is much more common. If the shots are safe, why is this happening?
- I have direct personal experience with the vaccine: two people I know were killed by the vaccine, none from COVID. I know many people who are vaccine injured from the COVID vaccine.
- Corruption in the VAERS system used to track adverse events. See this presentation by Albert Albert Benavides. In addition, the v-safe system showed that 8% of the people who got the vaccine had to see medical attention (which is in itself a train wreck), but the CDC refused to voluntarily disclose this important information and even today they don’t talk about it.
- The CDC covered up 770 safety signals. They didn’t tell the public about them at all. Not even hinting at them. A safety signal is very serious. To get one safety signal would be concerning. But to get 770 safety signals triggered (on 770 different adverse event types) and then not say anything to the public about it is a sure sign of a very corrupt public agency whose job is to protect the manufacturers, not the public.
- Ed Dowd’s book statistics. This very popular book (“Cause Unknown”) listed 500 who died unexpectedly. Ed didn’t know how many were unvaccinated. Only one person has come forward saying that one of the people in the book who died after the vaccines rolled out was unvaccinated.
- Prominent doctor/scientists switching sides. Paul Marik is one of the top intensivists in the world. After seeing many COVID vaccine injured patients, he changed his mind about the safety of vaccines. When he was not allowed to practice medicine consistent with his Hippocratic Oath, he resigned his position.
- The corruption with COVID protocols. The COVID hospital protocols likely caused 90% of the COVID deaths in hospitals. This led to Paul Marik resigning. See details in this article. Why are doctors forced to use hospital protocols that kill a huge percentage of patients instead of using their best judgment to save patients?
- This JAMA paper shows that COVID and influenza vaccines don’t work. Why are we pushing a vaccine where the statistics clearly show the vaccines don’t work?
- The consistency of the data. There have been no counter-anecdotes showing the vaccines are safe. I keep looking for one and come up empty.
- No debates with anyone prominent promoting the government narrative. Those who promote the narrative refuse to engage in any scientific discussions to resolve differences of opinion. This is similar to the question of whether vaccines cause autism: nobody who thinks it doesn’t is willing to engage in a public discussion about it to discuss the evidence. Why not resolve the issue through dialog? It isn’t resolved in the peer-review literature where half the papers say vaccines cause autism and the other half don’t. Why can’t we talk about it?
- Fear and intimidation tactics are used to silence dissent. Open debate would be more productive. But people are not allowed to hold or discuss views that go against the “consensus” or they will lose their jobs, their certifications, or their medical licenses. Health care workers are told they will be fired if they report an adverse event to VAERS, there are nurses who won’t talk about anaphylaxis after getting the vaccine for fear of being fired, vaccine injuries are covered up, hospital workers are afraid to talk about it at work.
- The cognitive dissonance is very disturbing. When healthcare workers bring up the topic of mortality and morbidity due to the vaccine, their peers say nothing and walk away.
- Censorship tactics employed by the US government to silence dissent instead of public recorded open debates. History has shown that purveyors of censorship are always on the wrong side of the issue.

- We have stopping condition. The Schwab paper showed people are being killed by the vaccine. The paper established that the rate of deaths was sufficient to halt the vaccine as unsafe. Nobody paid attention. The stopping condition is one death per million doses. So if you give 750M doses, you should have fewer than 750 deaths. The Schwab paper estimated that 14% of the people who died within 20 days of vaccination were killed by the vaccine. 14% of 137,000 people is 19,000 people which is more than 750 people.
- Highly respected scientists are calling for a halt to the vaccine. Peter McCullough has called for an end to the COVID shots, yet it falls on deaf ears. Peter McCullough and European Parliament 14 SEPT 23. Dr. Peter McCullough Calls For Complete Stop To All COVID Injections - Not Safe For Human Use: “I submit to you the COVID-19 vaccines and all of their progeny & future boosters are not safe for human use.”

- BA.1 and BA.2: first detected in February 2022.
- BA.4 and BA.5: first detected in May 2022.
- XBB.1.5 (Kraken): an offspring of two BA.2 sublineages first detected in October 2022.
- BA.2.86 (Pirola): first detected in 2023 and is currently being monitored.
- EG.5 (Eris): first detected in Feb 2023, peaked in October, and is now declining.
- HV.1: first detected in July 2023, has taken the lead in the United States at the end of October 2023.
The fierce battle between the virus and human technology has become a marathon. With each generation of vaccine development, who were the winners?
First Generation Vaccine: Delta Emerged, Creating Global Havoc
In January 2021, the original mRNA monovalent vaccines developed by Pfizer and Moderna and based on the old Wuhan strain were launched at a rocket-like speed.
In June 2021, when more than 50 percent of the U.S. population had received two doses of these vaccines, the stage was set for various mutants to take over, including the well-known alpha and delta variants.
A key mutation in spike protein called N501Y, which can escape from vaccine protection, was discovered in alpha. It was also found in two other major variants prevalent during that time and significantly increased in the rate at which it spread.
Shortly thereafter, Delta (B.1.617.2) emerged and presented even more enhanced transmissibility and vaccine escape ability with its intriguing spike protein double mutations of L452R and E484Q, refreshing the viral spreading and escaping records. It was designated as a "variant of concern" by the World Health Organization (WHO) on May 11, 2021.
These double mutations in the spike protein cause the vaccine-induced antibodies to significantly lose their ability to bind to delta, resulting in immunological evasion and causing major global havoc.
The increased binding affinity caused by delta makes it much easier to replicate in human cells. It was reported that patients infected with delta had a viral load 1000 times greater than patients with the original strain. It's also been able to spread twice as fast as the original SARS-CoV-2 virus.
In July 2021, preliminary data from Israel showed that Pfizer's vaccine efficacy was significantly reduced at five and six months after vaccination to 44 percent and 16 percent, respectively.
In a July 2021 outbreak in Massachusetts, 74 percent of breakthrough infections occurred in fully vaccinated persons, and the delta variant was detected in 90 percent of them.
The first round of the battle between the vaccine and the virus concluded with an overwhelming vaccine failure when the first generation of the COVID-19 mRNA vaccine met the unexpected delta variant.
Vaccine Versus Virus: The First Battle Round
Vaccine: Monovalent.
Result: The vaccine failed.
Time lapsed: Seven months from the first monovalent vaccine launched in January 2021 until the dominant delta wave in July 2021 in the United States.
Since then, a concern regarding the vaccine strategy of generating vaccine escape variants has been raised by scientists, including researchers from Michigan State University.
Second Generation Vaccine: XBB.1.5 Won
People continued to witness the declining effects of the original vaccine against delta, even after boosters were widely administered. The government continually stressed that the original vaccines had sufficient efficacy, one time after another.
Almost all of Omicron and its subvariants have developed specific mutations that have made them spread more quickly while evading our immune response. It has been clearly defined as an immune escape strain according to this Nature review.
A surprising virus, Omicron (B.1.1.529) surged more quickly than any previous strain and completely took over by April 2022. This emergence of hypermutated, increasingly transmissible Omicron variant significantly threatened the vaccine strategy.
It harbors multiple amino acid mutations in the spike (including Q498R and N501Y), which significantly enhance binding to the ACE2 receptor. It has also altered the cell entry pathway which further contributes to its ability to escape from vaccine protection.
In mid-2022, BA.4 and BA.5 lineages of Omicron were the dominant COVID-19 variants in the United States and were predicted to circulate in the second half of 2022.
Thus, Pfizer and Moderna quickly took the initiative to develop bivalent boosters based on the original strain from Wuhan and Omicron BA.4 and BA.5. They made it within another miraculously short time frame of just a few months.
On August 31, 2022, the FDA approved the bivalent booster shots of COVID-19 mRNA vaccines designed to target the Omicron subvariants BA.4 and BA.5, with Pfizer only providing the data on eight mice.
However, Omicron keeps quickly changing, splitting into even more diversified subgroups. Soon after the new bivalent vaccine was distributed, BA.4 and BA.5 became history.
A new variant XBB.1.5 began appearing in October 2022 and reached its peak in April 2023. It combines two descendent lineages (BA.2.10.1 and BA.2.75) of Omicron. The featured new spike protein mutation (F486P) leads to increased transmissibility and significant escape from the vaccine.
Not surprisingly, the antibody levels to XBB.1.5 in bivalent mRNA-boosted individuals declined significantly to pre-booster levels after only three months. The bivalent booster vaccine effectiveness against COVID-19-associated hospitalization declined to as low as 24 percent at six months post-vaccination, according to CDC data collected from September 2022 to April 2023.
The second round ended when the second generation bivalent mRNA vaccine encountered the XBB.1.5 starting in April 2023.
Vaccine Versus Virus: The Second Battle Round
Vaccine: Bivalent mRNA.
Result: The vaccine failed.
Time lapsed: Five months after the bivalent booster vaccine launched in September 2022 and was utilized until the U.S. dominant wave of XBB.1.5 in January 2023 emerged.
Vaccine Races with SARS-CoV-2 Variants (The Epoch Times)
Third Generation Vaccine: Doomed to Fail
As of September 2023, the Pfizer-BioNTech and Moderna mRNA vaccines have been reformulated—for the third time—this time based on XBB.1.5, which is the great-grandchild of Omicron. This latest booster recommendation applies to all individuals, regardless of previous COVID-19 vaccination history.
However, one month before the 3.0 vaccine was approved, the dominant virus had already changed from XBB.1.5 to EG.5—the "Eris" variant, which spreads faster and has a stronger ability to escape from the XBB.1.5 vaccine.
Vaccine Versus Virus: The Third Battle Round
Vaccine: XBB.1.5 mRNA.
Result: Vaccine doomed to fail.
Time lapsed: Less than one month from the XBB.1.5 booster vaccine launch in October 2023 to the U.S. dominant wave of vaccine escape by EG.5 or other cousin variants in October 2023.
Omicron continues to change from XBB to JN, HK.3, EG.5, and HV.1—all belonging to the huge and diversified Omicron family.
EG.5, carrying an additional F456L mutation, is significantly more resistant to neutralization by the sera from vaccinated people. That means even the most recent version of the COVID-19 vaccine based on XBB.1.5 is going to lose its protection with EG.5. Since the risk of breakthrough infection remains high, the WHO listed EG.5 as a "variant of concern" in early August.
While HV.1 shares almost all spike mutations that EG.5 carries, it took on a surprising additional mutation (L452R) from a remote ancestor delta variant in 2011, which had normally disappeared in the omicron variant. HV.1 can further escape the XBB.1.5-based vaccine-induced immunity and is even more evasive than EG.5.
The same detour trick of HV.1 is also used by JN.1 coming on the scene in August 2023. It gains an additional L455S mutation, switching from the XBB sublineage to BA.2.86 (Pirola).
The HK.3 virus has played a novel trick. It has two mutations in the adjacent spike 455 and 456 positions (L455F and F456L), thus called a "FLip." Together, this virus binds even more tightly to ACE2 and is taking off slowly in Brazil and Spain.
Both HK.3 (FLip) and JN.1 present even lower binding affinities, meaning the vaccine is even less effective than the current version, raising further concerns over vaccine strategy.
All these new variants are a direct result of the so-called vaccine-escaped viral mutations.
Despite the extraordinary speed of vaccine development against COVID-19 and the continued mass vaccination program, the never-ending emergence of new SARS-CoV-2 variants threatens to significantly overturn the vaccine's intended effects.
This is a tiring battle between vaccines and the virus. The winners and losers are clear. The microscopic tricks utilized by the SARS-CoV-2 virus variants are far superior to the vaccines' unproven technology.
Alerts have been raised and major concerns have been discussed by scientists as early as 2021 in top-ranked journals including The Lancet and Nature in addition to Nature Reviews, eBioMedicine (part of The Lancet Discovery Science), and other publications through 2023.
The common view is that the pressure exerted on viruses from repeated vaccination programs serves as a primary driver of the diversified variants of SARS-CoV-2.
If humans continue to develop vaccines based on these emerging new variants, there will continue to be repeated failures. How many more failures will it take to realize that all of these vaccine efforts have been in vain?
It is a time for rational deliberation to pause to reflect on finding the root cause of the viral infection.
We already have a dynamic shield of protection against serious viral attacks—our natural immunity. Only by facing our own innate immunity will the virus find its tricks useless.
- In all countries included in the analysis, all-cause mortality increased when COVID-19 vaccines were deployed.
- Nine of 17 countries had no detectable excess deaths following the World Health Organization’s March 11, 2020, declaration and the beginning of the COVID-19 vaccination campaign.
- Unprecedented peaks in all-cause mortality were observed in January and February 2022, during the summer season of Southern Hemisphere countries coinciding with or following the rollout of boosters in 15 of 17 countries studied.
- Excess all-cause mortality during the vaccination period beginning January 2021 was 1.74 million deaths, or one death per 800 injections, in the 17 countries studied.
- The vDFR increased exponentially with age, reaching almost 5 percent among those 90 years and older who received a fourth vaccine dose.
"There is no evidence in the hard data of all-cause mortality of a beneficial effect from the COVID-19 vaccine rollouts. No lives were saved,” Denis Rancourt, co-director of Correlation Research in the Public Interest with a doctorate in physics, told The Epoch Times in an email. “On the contrary, the evidence can be understood in terms of being subjected to a toxic substance. The risk of death per injection increases exponentially with age. The policy of prioritizing the elderly for injection must be ended immediately.”
Peaks in All-Cause Mortality Coincide with Booster Doses
Using mortality and vaccination data from Chile and Peru by age and dose number, researchers observed clear peaks in all-cause mortality in July through August 2021, January through February 2022, and July through August 2022 among elderly age groups. The increase in all-cause mortality observed in January and February 2022 in both countries coincided with the rapid rollout of Chile’s fourth COVID-19 vaccine dose and Peru’s third dose.
It is unlikely that the rise in all-cause mortality coinciding with the rollout and sustained administration of COVID-19 vaccines in all 17 countries could be due to any cause other than the vaccines, researchers said.
In Chile and Peru, the vDFR increased exponentially with age and was most significant for the most recent booster doses, resulting in one death per 20 injections of vaccine dose for in those over age 90. This pattern was similar to data the same researchers collected in Australia.
“Synchronicity between the many peaks in ACM (in 17 countries, on 4 continents, in all elderly age groups, at different times) and associated rapid booster rollouts allows this firm conclusion regarding causality and accurate quantification of COVID-19-vaccine toxicity,” the researchers wrote.
Results in other countries mirrored what was observed in Chile and Peru in every case where age-stratified mortality and age-stratified dose-specific vaccination data were available. In 15 countries with sufficient mortality data, an unprecedented surge in all-age all-cause mortality during or near January and February 2022 coincided with or was immediately preceded by a rapid rollout of booster doses three or four depending on the country and the continued administration of non-booster doses.
Researchers Found No Evidence COVID-19 Vaccines Improved Mortality
The researchers said their findings are conclusive, and the associations observed are numerous and systematic. They could not find a single counter-example showing COVID-19 vaccines improved all-cause mortality.
“If vaccines prevented transmission, infection or serious illness, then there should be decreases in mortality following vaccine rollouts, not increases, as in every observed elderly age group subjected to rapid booster rollouts. And, mortality would not increase solely when vaccines are rolled out, where no excess mortality occurs before vaccine rollouts, as we have documented here, in nine countries across three continents,” researchers concluded.
According to the report, data from numerous countries such as India, Australia, Canada, Israel, and the United States show a similar phenomenon—abnormal peaks in all-cause mortality coinciding with booster rollouts. In the United States, deaths were prominent in the 25 to 64 age group in 21 states, coinciding with a “rapid surge” in vaccines given during the “vaccine equity” campaigns launched by regulatory agencies. Researchers estimated the United States experienced roughly 160,000 excess deaths during a period where more than 60 million COVID-19 vaccine doses were administered.
- Genotoxicity.
- Genome integration.
- Germ-line transmission.
- Insertional mutagenesis.
- Tumorigenicity.
- Embryo/fetal and perinatal toxicity.
- Long-term expression.
- Repeated toxicity.
- Excretion in the environment, such as shedding through seminal fluid or breast milk.
“The long-term safety monitoring of GTPs is required over several years whereas, for vaccines, it is generally only carried out over a few weeks,” wrote Dr. Helene Banoun with the French Institute of Health and Medical Research in the paper. “This should not be acceptable, given the persistence of the drug product and the expressed protein.”
In addition, known results of anti-cancer therapies that use gene therapy technology and mRNA vaccines could lead us to anticipate safety and efficacy problems, she added.
In the EU, gene therapy medicinal products are required to undergo “tests or trials to evaluate the risk of genome integration and germ-line transmission, even if this integration is unlikely,” and tests and clinical trials to evaluate the risk of “insertional mutagenesis, tumorigenicity, embryo/fetal and perinatal toxicity, and long-term expression.”
EMA requires “extensive studies on both the nucleic acid and the vector particle/delivery system that includes biodistribution, dose study, potential target toxicity, the identification of the target organ to obtain biological activity, toxicity linked to the expression of structurally altered proteins.”
It is necessary to insist pharmacokinetic studies be performed to determine how the body interacts with the administered substance during the entire duration of exposure—even though they are generally not required for vaccines unless there’s a new formulation or a vaccine contains novel adjuvants or excipients (inactive substances such as preservatives).
For GTPs, shedding studies are also needed to determine excretion and dissemination in the body, and biodistribution studies are needed to assess where injected compounds—such as lipid nanoparticles, the delivery system used to deliver mRNA—travel in the body and which tissues or organs they accumulate in.
After assessing Pfizer and Moderna’s COVID-19 vaccine documents obtained by attorney Aaron Siri through the Freedom of Information Act, Wiseman noted many studies listed in nonclinical summaries that should have been performed but were not.
“Several studies should have been done but weren’t done because they fell under the auspices of vaccines. But if you read the guidelines, it doesn’t say these studies are unnecessary, just that circumstances may deem them unnecessary,” Wiseman said. “We need laws for products that say you can’t just exclude them from regulations because you feel like it—because they are still gene therapies,” he said.”We are hijacking the machines of our bodies to produce spike proteins in an uncontrolled, undefined way—there are too many things we don’t know about.”
Some countries have recently halted or slowed down recommendations for COVID-19 vaccination after years of promoting repeated shots as data show the vaccines provide substandard protection against infection and short-lived protection against severe illness. The United States, for instance, stopped recommending boosters for all and changed the primary vaccination of the Moderna and Pfizer vaccines from two doses to one.
Still, some health agencies are moving toward a model based on the approach to influenza vaccination. That would involve selecting updated vaccine compositions each year aimed at targeting the circulating COVID-19 strains, and recommending certain groups, or virtually everybody, get an annual shot.
The World Health Organization said in May that the composition should be updated to focus on the XBB.1 Omicron subvariant “in order to improve protection.” Advisers to the U.S. Food and Drug Administration are set to convene in June to consider whether the vaccines should be updated for the 2023–2024 “vaccination campaign.” Officials in many countries have already discontinued the old Moderna and Pfizer vaccines and cleared shots that target the BA.1 or BA.4/BA.5 Omicron subvariants.
Africa Is Starkly Unvaccinated, And Starkly Unvanquished By COVID
MONDAY, JAN 23, 2023 - 04:30 AM
Authored by Colleen Huber via The Epoch Times,
Let's study that victory with utmost diligence...
Africa as a Whole Is Very Strikingly Unvaccinated, According to Johns Hopkins University, Our World in Data.
Let’s keep in mind that most striking continent on an otherwise bleak world map, as we examine the following map, which shows Africa’s burden of COVID cases since the beginning of COVID.
Here is Africa’s relative share of COVID cases since the beginning of COVID:
https://coronavirus.jhu.edu/map.html
The Data Reports That Can Be Expected Three Years Into a Pandemic
One would reasonably expect a worldwide pandemic that began three years ago to have been recorded with some ballpark accuracy in case counts, and morbidity and mortality data throughout the world by now, as each hemisphere has been through three winters. One would also expect that a worldwide vaccine campaign that peaked over a year ago to have resulted in reliable vaccine uptake maps. One would expect a general consensus regarding such data. So let’s accept the above maps as not (or not yet) disputed, and as reliable documentation of historical events of pinnacle importance, events that behoove humanity to understand well, and to understand as thoroughly as if our future well-being depends on it.
One who has faith in the practice of vaccination would have also expected that vaccines carrying the name of the pandemic to have mitigated case counts of the same disease. How then is the overall experience of the African continent to be understood?
Africa was not the only part of the world where reported COVID cases have been low. Prior to vaccination, numerous countries were barely impacted at all by COVID. Let’s zoom out from Africa now to examine events in other countries.
Former US Dept of Justice adviser Gavin de Becker wrote an article on Children’s Health Defense [3] that also appears in a book by Edward Dowd, Cause Unknown; in it he looks at COVID mortality in various nations, primarily in Asia, but also in Africa, Europe, Latin America and the Middle East, after COVID began, as well as before and after the launch of their vaccination campaigns. Three of de Becker’s timelines are as follows. De Becker indicates with a syringe pointer the date at which each of the following countries began their COVID vaccine campaigns.
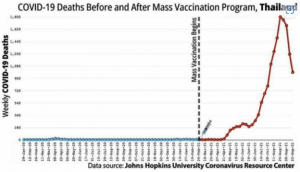
Gavin de Becker, https://childrenshealthdefense.org/defender/covid-vaccine-deaths-cause-unknown/
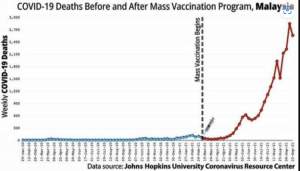
Gavin de Becker, https://childrenshealthdefense.org/defender/covid-vaccine-deaths-cause-unknown/
Gavin de Becker, https://childrenshealthdefense.org/defender/covid-vaccine-deaths-cause-unknown/
De Becker notes that “the reality displayed on the graphs you’ve seen is undeniable, cannot be unseen, and is available to anyone more interested and more industrious than media and governments have been.”
Elusive Truth in Morbidity and Mortality Data: The PCR Problem
De Becker’s article, as the Johns Hopkins data, necessarily relies on reports that are fraught with much difficulty, for the reasons I review below, primarily the wildly misapplied PCR “test” to COVID diagnosis. However, because that alleged test is primarily how the world has evaluated and tallied COVID cases and deaths for three years, we are necessarily dependent on and limited to the derived data from this alleged test for any meaningful assessment of COVID epidemiology.
COVID-19 diagnoses have been troublesome from the beginning. It has been noted, including at Johns Hopkins University, which produces the most university-based statistical data on COVID, that reported deaths from flu, pneumonia, heart disease and diabetes decreased significantly in 2020, while COVID-19 deaths became the cause of death listed for now over six million lost lives around the world. Flu and pneumonia as primary causes of death nearly disappeared. For every lost life and every grieving family, the signs and symptoms of this respiratory disease phenomenon occurred, and then it is a matter of disagreement as to whether we will call those deaths flu, pneumonia or COVID, with no particular loss of life any less tragic for the bereaved from one diagnosis from the others. Cardiovascular mortality reports also dropped precipitously, without any credible reason for the change. Another unexplained surprise to epidemiologists was that those deceased with a COVID cause of death exceeded the average age of life expectancy in the US. Genevieve Briand of Johns Hopkins University discusses these anomalies.
Flu and pneumonia had always been among the most threatening diseases for seniors. And then the mortality reports changed. There are two major influences that created an alleged 2020 pandemic out of what was otherwise a typical flu year. The following two factors led to false reporting of US mortality data for COVID:
First Domino Falls
The first was a manufacturing technique that wound up being wildly misappropriated as a diagnostic test, despite the prior protests of its inventor, the late Kary Mullis, PhD. The essence of the world’s confusion and fear of COVID stems from the testing itself. Reverse-transcriptase, polymerase chain reaction (RT-PCR) is a method for producing more RNA nucleic acid sequences. Essentially, PCR does what it was designed by Mullis to do: It matches or aligns specific genetic signatures between a given test reagent and a sample. As the test is run in consecutive cycles, each cycle multiplies the sample. So that sample then grows exponentially. The PCR is simply incapable to determine if the introduced sample contains adequate viral particles or virions to rise to the threshold of causing an infection.
For those who have worked with PCR, it is understood that any PCR process run through 20 or more cycles is useless for detection. The CDC acknowledged that 33 cycles or more are unlikely to detect active virus. Yet for all of 2020, throughout the US, the number of cycles used in “COVID-19 testing” have been above 37 and often well into the 40’s. Boris Borovoy and I discuss problems related to this misuse of PCR. The misplaced faith in this manufacturing technique as a test of anything having to do with contagion was the misjudgment at the core of worldwide disaster.
From such a simple decision and widespread acquiescence to create a test out of a non-test, whether by error, misunderstanding or possibly worse on the part of some: deliberate misuse of an industrial process, a new world may be in its birth from this practice. This misuse, born of widespread misunderstanding of PCR, became the pretext for the estimated four trillion dollar COVID industry.
Second Domino Falls
The second factor that fired up the COVID engines, so to speak, at least in the United States, was the financially-incentivized COVID cause of death. Under the US CARES Act, hospitals were compensated more than twice as much money for a COVID case than a flu or pneumonia case, and the most lethal treatments were compensated even further. Many US hospitals made millions of dollars from this shift in diagnosis during treatment and on death certificates.
Other forensic evidence shows lack of a pandemic in 2020. Wall Street seems to need and to have greater reliance on accurate data than governments. COVID is primarily a pathogenic disease of the respiratory tract, with dyspnea (shortness of breath) noted as one of the most common symptoms along with coughing, in which acute and late-stage care often involves supplemental oxygen. Oxygen use would be the most reliable artifact of COVID care. Therefore, we looked at sales of medical oxygen, by revenue of the top companies that produce it, in 2020 vs 2019. We then noted that their sales decreased in that time. Meanwhile, sales by six of the top oxygen concentrator producers trading on the NYSE had increased by less than one percentage point from 2019 to 2020. This is the 0.93% in the last line of the following table. In the same time, the world’s population grew by 1.05%.
C Huber, B Borovoy. Data that disprove the COVID-19 pandemic. Dec 19, 2020. PDMJ. https://pdmj.org/papers/is_there_a_pandemic
For whatever other wealth distribution occurred during what is widely considered to be the peak pandemic year of 2020, the New York Stock Exchange does not reflect the primary medical need of the pandemic patients to have made impact on the revenue of the main companies supplying that medical demand.
How Africa Defeated COVID so Decisively Without Vaccines
Part of the African continent’s success is no doubt due to a fortunate accident of microbiology, infectious diseases, pharmacology and immunology. It so happens that two of the most effective treatments for COVID, ivermectin and hydroxychloroquine, are also routine prophylactic weekly medicines throughout equatorial Africa, because they happen to be known for a half-century as the most effective, applicable and safest anti-parasite medications. So the population, particularly through about 31 countries, the tropical middle rectangle roughly, of Africa already were well-equipped prior to COVID events launching in late 2019 to early 2020.
As fortune would have it, the unpatented and relatively inexpensive half-century old drug ivermectin, whose inventors won the Nobel Prize for Medicine in 2015, also has been the most effective medicine against COVID, [15] due in part to its specific effect against RNA transcriptase, as well as its blocking effect on all three parts of the trimeric spike protein, and other mechanisms.
Hydroxychloroquine is also used widely throughout at least equatorial regions of Africa as a prophylactic against parasites, but which fortunately has now been studied extensively and used successfully as both prevention and treatment of COVID disease, and as inhibitor of SARS-CoV-2 replication and activity. This is shown in over 380 studies conducted in 55 countries.
Africa Leads Again
This is not the first piece of evidence that Africa is leading the world away from a microbial-pretext tyranny. Last summer, Africa stood alone in being the continent, led by Botswana, to pull the worlds’ people back from the precipice, while pushing the World Health Organization (WHO) back from their attempted tyranny over all world governments. [18] This danger is by no means past, and new efforts for WHO dominance over the world are ominously re-grouping at this time.
Africa led the way and inspires the world. Are the politicians and “public health experts” of the rest of the world humble enough to admit their grotesque errors, even crimes, and to learn from the peoples of the African nations, their experiences and lessons on handling a pandemic?
Or will ethnocentrism or a hostile and racist pride, or the sheer greed stimulated by the lucrative COVIDmania boondoggle, prevent the rest of the world’s willingness to learn from the African experience? Will such provincial and purchased attitudes bury the 21st century’s most important lesson to date?
They forgot to tell you: the new bivalent vaccines make it MORE likely you'll get COVID
The more boosters you get, the more depressed your immune system becomes. That's why every time they inject you, it makes you more vulnerable to getting COVID.

Executive summary
The data I’ve been able to collect is consistent with the hypothesis that the more boosters you get, the worse the outcome.
Even the Moderna data was consistent with that.
Introduction
I did a survey of hospitals on January 12, 2023 and got 101 responses.
Only one hospital, The Wayne Center (nursing home) Wayne PA, did they think the booster made things slightly better. In 10 places, it made NO DIFFERENCE. Everywhere else, the reporters believed it made things worse.
In 71 places, they believed it made things MUCH WORSE.
But wait… didn’t the government agencies say it reduces your risk of death from COVID by 10X?
If that is true, then how come I can’t find any nursing home in the world where that happened? It’s easy to find places where the all-cause mortality (ACM) skyrocketed after the shot (one Australian nursing home in Clifton Hill lost over 20% of their residents in 12 months after the shots rolled out), but I can’t seem to find a success story where the ACM plummeted after the shots. Odd, isn’t it?
Moderna data that was initially withheld from the FDA
More troubling is that Moderna decided not to share with the FDA panel information that is consistent with my hypothesis that more boosters made you more likely to be infected with COVID.
Find this hard to believe? This is a direct quote from the CNN article entitled FDA vaccine advisers ‘disappointed’ and ‘angry’ that early data about new Covid-19 booster shot wasn’t presented for review last year which said:
The data that was not presented to the experts looked at actual infections: who caught Covid-19 and who did not.
It found that 1.9% of the study participants who received the original booster became infected. Among those who got the updated bivalent vaccine – the one that scientists hoped would work better – a higher percentage, 3.2%, became infected. Both versions of the shot were found to be safe.
In other words, when the data shows the vaccines aren’t working, the drug companies do the only ethical (in their mind) thing: they withhold the data from the FDA panel.
Paul Offit said he’s not getting the bivalent booster. He said there is no benefit and it’s all risk. For once, I agree with Paul.
If Paul Offit isn’t taking the new bivalent booster, why should you?
Answer: You shouldn’t. Nobody should.
Will anyone from the drug companies debate any of us on whether more boosters are both safe and beneficial?
No way. They’d lose.
The drug companies don’t stand behind their product. They don’t need to because:
- they have liability protection (why do they need that again???) and
- because they have conned lawmakers all over the world into coercing people to take the vaccine.
The only thing a debate would do is expose that they are lying about the safety and efficacy of the vaccines. That’s why none of them will take my $1M bet (with a term sheet similar to this one I negotiated with Saar Wilf for $500K) and show the world I’m wrong. Because they know they will lose and that will end their revenue stream.
I’m willing to bet anyone $1M I’m right on any of these topics:
- The mRNA vaccines in the US have killed more people than they’ve saved
- The three randomized trials have not shown that masks make a measurable positive difference in protecting people from getting COVID
- The mRNA COVID vaccines cause strokes
- The mRNA vaccines cause measurable heart injury in >2% of people injected
- The vaccines have seriously injured over 500,000 people in America
- More boosters are safe and more beneficial (more is better)
You can accept any of these bets here if you are serious about negotiating a term sheet similar to this term sheet.
Summary
In short, for the drug companies, I’m willing to lower the bet to a dime just to prove that they won’t even risk a dime of their money. But risking your life? No problem! They have no liability!
Think about it.
You should be very upset about this.
Confidential Pfizer and Government Documents confirm ADE, VAED, and AIDS due to COVID-19 Vaccination leading to Millions “Dying Suddenly” & still counting
BY THE EXPOSÉ ON DECEMBER 30, 2022
Confidential documents reveal that within months of receiving the initial doses of the COVID-19 vaccine, some individuals are developing Antibody-dependent enhancement (ADE) and Vaccine-Associated Enhanced Disease (VAED).
And as if that weren’t alarming enough, official documents also prove that a mysterious form of Acquired Immune Deficiency Syndrome is also appearing in a disturbing number of recipients just five months after their initial injections.
This may explain why, tragically, official Government records confirm that millions of people have mysteriously died suddenly in countries around the globe, including the United States, United Kingdom, Australia, Canada, and Europe, in the wake of the widespread distribution of the COVID-19 vaccines.
Antibody-dependent enhancement (ADE) and Vaccine-Associated Enhanced Disease (VAED). are serious adverse events that can occur after vaccination.
ADE and VAED can occur when an individual is exposed to a pathogen, such as the alleged Covid-19 virus, after receiving a vaccine that does not provide full immunity.
In these cases, the vaccine-induced antibodies may actually enhance the ability of the pathogen to infect cells, leading to more severe illness than if the individual had not received the vaccine.
When a vaccine causes ADE or VAED, it can have significant public health implications.
First and foremost, individuals who receive the vaccine and develop ADE or VAE may suffer from severe illness, and in some cases, even death.
One example of a previous vaccine that has been associated with ADE is the dengue vaccine. In many cases, individuals who received the dengue vaccine and were subsequently infected with the dengue virus experienced more severe illness and an increased risk of hospitalization and death.
Similarly, ADE has been reported in individuals who received vaccines for respiratory syncytial virus (RSV) and HIV.
One example of a bacterial infection that could potentially be worsened by ADE or VAE is streptococcus A (strep A) infection. Strep A is a type of bacteria that can cause a wide range of illnesses, including sore throat, pneumonia, and sepsis. You will have most likely seen in the mainstream news that Strep A infection is killing children left, right and centre this winter.
Covid-19 vaccination is the most likely reason for this, and the most important piece of evidence to support this fact is official Government reports that prove Covid-19 vaccination damages the immune system and has the potential to cause some new form of acquired immunodeficiency syndrome.
It is entirely possible that ADE and VAED can lead to immense immune system damage similar to that seen in acquired immune deficiency disorder (AIDS).
In individuals with AIDS, the immune system is severely compromised, making them more susceptible to infections and other diseases. Similarly, the occurrence of ADE or VAED can lead to damage to the immune system, potentially leading to a higher risk of infections and other diseases.
In addition to the risk of severe illness comparable to AIDS, ADE and VAED may also increase the risk of developing certain cancers. For example, some studies have suggested that ADE may increase the risk of developing certain types of lymphoma.
The occurrence of these adverse events has had devastating consequences for the individuals who develop them and confidential and official documents prove that ADE and VAED have occurred due to COVID-19 vaccines, leading to a new form of Acquired Immune Deficiency Syndrome and millions of excess deaths around the world.
Pfizer, the company hit with the largest healthcare fraud settlement and criminal fine to date in 2009; which also happens to be the same company behind the first ever mRNA gene therapy injection administered to the general public under emergency use authorisation in the name of Covid-19, has admitted in confidential documents, that it desperately tried to keep from going public, that its Covid-19 mRNA gene therapy may cause Vaccine-Associated Enhanced Disease.
The US Food and Drug Administration (FDA) attempted to delay the release of Pfizer’s COVID-19 vaccine safety data for 75 years despite approving the injection after only 108 days of safety review on December 11th, 2020.
The FDA originally said that they were prepared to release 500 pages per month in a response to the Freedom of Information (FOI) request filed on behalf of Public Health and Medical Professionals for Transparency (PHMPT) requesting the safety data.
Instead, in early January 2022, Federal Judge Mark Pittman ordered them to release 55,000 pages per month. They released 12,000 pages by the end of January.
Since then, PHMPT has posted all of the documents to its website.
One of the documents contained in the latest data dump is ‘reissue_5.3.6 postmarketing experience.pdf’. Table 5, found on page 11 of the document shows an ‘Important Potential Risk’, and that risk is listed as ‘Vaccine-Associated Enhanced Disease (VAED), including Vaccine-Associated Enhanced Reporatory Disease (VAERD)’.

Pfizer claimed in their confidential document that up to 28th Feb 2021, they had received 138 cases reporting 317 potentially relevant events indicative of Vaccine-Associated Enhanced Disease. Of these 71 were medically significant resulting in 8 disabilities, 13 were life-threatening events, and 38 of the 138 people died.
Of the 317 relevant events reported by 138 people, 135 were labelled as ‘drug ineffective’, 53 were labelled as dyspnoea (struggling to breathe), 23 were labelled as Covid-19 pneumonia, 8 were labelled as respiratory failure, and 7 were labelled as seizure.
Pfizer also admitted that 75 of the 101 subjects with confirmed Covid-19 following vaccination, had severe disease resulting in hospitalisation, disability, life-threatening consequences or death.
But Pfizer still definitively concluded, for the purposes of their submitted safety data to the Food and Drug Administration (FDA), the very data that was needed to gain emergency use authorisation and make them billions and billions of dollars, that ‘None of the 75 cases could be definitively considered as VAED’.
But Pfizer then went on to confirm that based on the current evidence, VAED remains a theoretical risk.
This confidential data proves that the Covid-19 injections should never have been granted emergency use authorisation, and should have been pulled from distribution by the FDA as soon as they sighted the figures.
And here’s how we know the ADE and VADE caused by Covid-19 vaccination have led to immune system damage comparable to AIDS.
The UK Health Security Agency (UKHSA) used to publish a weekly Vaccine Surveillance Report, with each report containing four weeks worth of data on Covid-19 cases, hospitalisations, and deaths by vaccination status.
We analysed 5 of these published Vaccine Surveillance Reports containing data from August 16th 2021 to January 2nd 2022, in order to get a clear picture of the effect the Covid-19 vaccines were having on the immune systems of the vaccinated population, and this is what we found…
The UKHSA Vaccine Surveillance Reports used for our investigation can all be found here –
- ‘Covid-19 Vaccine Surveillance Report – Week 37’ (Published by PHE)
- ‘Covid-19 Vaccine Surveillance Report – Week 41’ (Published by UKHSA)
- ‘Covid-19 Vaccine Surveillance Report – Week 45’ (Published by UKHSA)
- ‘Covid-19 Vaccine Surveillance Report – Week 49’ (Published by UKHSA)
- ‘Covid-19 Vaccine Surveillance Report – Week 1 – 2022’ (Published by UKHSA)
You were told that the Pfizer Covid-19 mRNA injection had a vaccine effectiveness of 95%.
The following graph illustrates the increase/decrease in vaccine effectiveness by the month among each age group over a period of 5 months from 16th Aug 21 to 2nd Jan 22.
The first booster shots were administered in week 37 of 2021, and this graph illustrates clearly how they provided a boost in vaccine effectiveness in the following two months. But unfortunately, it also shows how short-lived this boost was with the effectiveness of the Covid-19 vaccines falling to frightening levels between weeks 49 and 52.
Real-world vaccine effectiveness dropped to the lowest levels yet across all age groups except for the over 70’s between December 6th and January 2nd, but the over 70’s still dropped into negative effectiveness.
The expected further boost to 40 to 69-year-olds did not materialise and instead, a huge tumble in vaccine effectiveness was recorded, dropping to -151% in 40-49-year-olds.
Vaccine effectiveness also tumbled in the 30-39-year-old age group to minus-123%, despite the booster jab being administered to millions in week 49.
That’s a far cry from the alleged 95% effectiveness you were told about.
But what does a positive/negative vaccine effectiveness actually mean?
Vaccines work by simulating a viral attack and provoking the immune system into responding as if you have had the virus. They are supposed to train the immune system to the point where you develop natural immunity to the virus.
Therefore, vaccine effectiveness is really a measure of the immune system performance of the vaccinated compared to the immune system performance of the unvaccinated.
The data published by the UKHSA confirms that the real-world effectiveness of the Covid-19 injections (really a measure of immune system performance) wains significantly in a short amount of time.
But, unfortunately for the vaccinated population, rather than the immune system returning to the same state it was prior to vaccination, the immune system performance begins to rapidly decline, making 3it inferior to that of the unvaccinated.
However, the UK Government data does prove that a booster dose of the vaccine can give a short-term boost to the immune system.
But, unfortunately, this same data shows that the immune system performance then begins to decline even faster than it has been prior to the booster dose being given.
This data, therefore, suggests that the vaccinated population will now require an endless cycle of booster shots to boost their immune systems to a point where it does not fail but is inferior to that of the unvaccinated population.
ADE or VAED can also lead to increased excess deaths, which are deaths that occur above and beyond the expected number of deaths in a population.
This is because ADE and VAED may cause more severe illness and an increased risk of death, leading to a higher number of deaths than would be expected in a population that has not been exposed to the vaccine.
This may explain why, tragically, official Government records confirm that millions of people have mysteriously died suddenly in countries around the globe in the wake of the widespread distribution of the COVID-19 vaccines.
Official reports published by the Governments of the USA, Canada, Australia, New Zealand, the UK & most of Europe, confirm 1.8 million excess deaths have been recorded since the mass roll-out of the Covid-19 injections.
Some countries have been quite transparent in publishing data on deaths, such as the UK and Europe for example. However, they have refused to actively publicise the figures because of what they reveal.
But other countries, such as the USA, have done their utmost to conceal data on deaths as much as possible.
However, we have finally managed to find the data for 15% of the world’s countries hidden deep within the website of an organisation known as the Organisation for Economic Co-Operation and Development (OECD).
The OECD is an intergovernmental organisation with 38 member countries founded in 1961 to stimulate economic progress and world trade. And for some reason, they host a wealth of data on excess deaths. You can find that data for yourself here.
The following chart reveals what we found in terms of excess deaths across the ‘Five Eyes’; which is an intelligence alliance comprising of Australia, Canada, New Zealand, the United Kingdom, and the United States, and a further 27 countries across Europe.


In conclusion, antibody-dependent enhancement (ADE) and vaccine-associated enhanced disease (VAED) are serious adverse events that can occur after Covid-19vaccination.
When a Covid-19 vaccinated individual is exposed to a pathogen all hell can break loose. In these cases, the vaccine-induced antibodies may actually enhance the ability of the pathogen to infect cells, leading to more severe illness than if the individual had not received the vaccine.
The occurrence of ADE or VAED is having significant consequences for both individual and public health.
It has led to severe illness and an increased risk of death for individuals who develop ADE or VAED, as well as an increased risk of developing certain cancers.
The occurrence of ADE or VAED has also led to increased excess deaths, with over 1.8 million in the ‘Five Eyes’ countries alone.
Vaccine manufacturers and Public Health agencies should have carefully monitored the public for any adverse events following vaccination and should have taken appropriate action to address the issues that have arisen.
This could have included revising vaccine recommendations, issuing warnings or alerts, and in some cases, withdrawing the vaccine from the market.
But they have done none of these things.
Which leaves us with the gnawing question of, why?
CDC Finally Releases VAERS Safety Monitoring Analyses For COVID Vaccines
MONDAY, JAN 09, 2023 - 03:00 AM
Authored by Professor Josh Guetzkow via Jackanapes Junction (some emphasis ours),
SUMMARY
- CDC’s VAERS safety signal analysis based on reports from Dec. 14, 2020 – July 29, 2022 for mRNA COVID-19 vaccines shows clear safety signals for death and a range of highly concerning thrombo-embolic, cardiac, neurological, hemorrhagic, hematological, immune-system and menstrual adverse events (AEs) among U.S. adults.
- There were 770 different types of adverse events that showed safety signals in ages 18+, of which over 500 (or 2/3) had a larger safety signal than myocarditis/pericarditis.
- The CDC analysis shows that the number of serious adverse events reported in less than two years for mRNA COVID-19 vaccines is 5.5 times larger than all serious reports for vaccines given to adults in the US since 2009 (~73,000 vs. ~13,000).
- Twice as many mRNA COVID-19 vaccine reports were classified as serious compared to all other vaccines given to adults (11% vs. 5.5%). This meets the CDC definition of a safety signal.
- There are 96 safety signals for 12-17 year-olds, which include: myocarditis, pericarditis, Bell’s Palsy, genital ulcerations, high blood pressure and heartrate, menstrual irregularities, cardiac valve incompetencies, pulmonary embolism, cardiac arrhythmias, thromboses, pericardial and pleural effusion, appendicitis and perforated appendix, immune thrombocytopenia, chest pain, increased troponin levels, being in intensive care, and having anticoagulant therapy.
- There are 66 safety signals for 5-11 year-olds, which include: myocarditis, pericarditis, ventricular dysfunction and cardiac valve incompetencies, pericardial and pleural effusion, chest pain, appendicitis & appendectomies, Kawasaki’s disease, menstrual irregularities, vitiligo, and vaccine breakthrough infection.
- The safety signals cannot be dismissed as due to “stimulated,” exaggerated, fraudulent or otherwise artificially inflated reporting, nor can they be dismissed due to the huge number of COVID vaccines administered. There are several reasons why, but the simplest one is this: the safety signal analysis does not depend on the number of reports, but whether or not some AEs are reported at a higher rate for these vaccines than for other non-COVID vaccines. Other reasons are discussed in the full post below.
- In August, 2022, the CDC told the Epoch Times that the results of their safety signal analysis “were generally consistent with EB [Empirical Bayesian] data mining [conducted by the FDA], revealing no additional unexpected safety signals.” So either the FDA’s data mining was consistent with the CDC’s method—meaning they "generally" found the same large number of highly alarming safety signals—or the signals they did find were expected. Or they were lying. We may never know because the FDA has refused to release their data mining results.
INTRODUCTION
Finally! Zachary Stieber at the Epoch Times managed to get the CDC to release the results of its VAERS safety signal monitoring for COVID-19 vaccines, and they paint a very alarming picture (see his reporting and the data files here, or if that is behind a paywall then here). The analyses cover VAERS reports for mRNA COVID vaccines from the period from the vaccine rollout on December 14, 2020 through to the end of July, 2022. The CDC admitted to only having started its safety signal analysis on March 25, 2022 (coincidentally 3 days after a lawyer at Children’s Health Defense wrote to them reminding them about our FOIA request for it).
[UPDATE: T Coddington left a link in comments to a website where he made the data in the Excel files more accessible.]Like me, you might be wondering why the CDC waited over 15 months before doing its first safety signal analysis of VAERS, despite having said in a document posted to its website that it would begin in early 2021—especially since VAERS is touted as our early warning vaccine safety system. You might also wonder how they could insist all the while that the COVID-19 vaccines are being subjected to the most rigorous safety monitoring the world has ever known. I’ll come back to that later. First I’m going to give a little background information on the analysis they did (which you can skip if you’re up to speed) and then describe what they found.
BACKGROUND ON SAFETY SIGNAL ANALYSIS
Back in June 2022, the CDC replied to a Freedom of Information Act (FOIA) request for the safety signal monitoring of the Vaccine Adverse Events Reporting System (VAERS)—the one it had said it was going to do weekly beginning in early 2021. Their response was: we never did it. Then a little later they said they had been doing it from early on. But by August, 2022, they had finally gotten their story straight, saying that they actually did do it, but only from March 25, 2022 through end of July. You can get up to speed on that here.
The analysis they were supposed to do uses what’s called proportional reporting ratios (PRRs). This is a type of disproportionality analysis commonly used in pharmacovigilance (meaning the monitoring of adverse events after drugs/vaccines go to market). The basic idea of disproportionality analysis is to take a new drug and compare it to one or more existing drugs generally considered safe. We look for disproportionality in the number of adverse events (AEs) reported for a specific AE out of the total number of AEs reported (since we generally don't know how many people take a given drug). We then compare to existing drugs considered safe to see if there is a higher proportion of particular adverse events reported for the new drug compared to existing ones. (In this case they are looking at vaccines, but they still use PRR even though they generally have a much better sense of how many vaccines were administered.)
There are many ways to do disproportionality analysis. The PRR is one of the oldest. Empirical Bayesian data mining, which was supposed to be done on VAERS by the FDA, is another. The PRR is calculated by taking the number of reports for a given adverse event divided by the total number of events reported for the new vaccine or the total number of reports. It then divides that by the same ratio for one or more existing drugs/vaccines considered safe. Here is a simple formula:

So for example, if half of all adverse events reported for COVID-19 vaccines and the comparator vaccine(s) are for myocarditis, then the PRR is 0.5/0.5 = 1. If one quarter of all AEs for the comparator vaccine are for myocarditis, then the PRR is 0.5/0.25 = 2.
Traditionally, for a PRR to count as a safety signal, the PRR has to be 2 or greater, have a Chi-square value of 4 or greater (meaning it is statistically significant) and there has to be at least 3 events reported for a given AE. (This also means that if there are tons of different AEs reported for COVID vaccines that have never been reported for any other vaccine, it will not count as a safety signal. I found over 6,000 of those in my safety signal analysis from 2021.
Of course a safety signal does not necessarily mean there is a problem or that the vaccine caused the adverse event. But it is supposed to set off alarm bells to prompt closer inspection, as in this CDC pamphlet:

Ah yes, shared with the public — after first refusing to share the results and months of foot-dragging following repeated FOIA requests! We will see that the CDC has not done a more focused study on almost any of adverse events with “new patterns” (AKA safety signals).
SO WHAT DID THE CDC ACTUALLY DO?
The Epoch Times obtained 3 weeks of safety signal analyses from the CDC for VAERS data updated on July 15, 22 and 29, 2022. Here I will focus on the last one, since there is very little difference between them and it is more complete. The safety signal analysis compares adverse events1 reported to VAERS for mRNA COVID-19 vaccines from Dec. 14, 2020 through July 29, 2022 to reports for all non-COVID vaccines from Jan 1, 2009 through July 29, 2022.
PRRs are calculated separately for 5-11 year-olds, 12-15 year-olds and 18+ separately. For each age group, there are separate tables for AEs from all reports, AEs from reports marked serious and AEs from reports not marked as serious.2 Recall that a serious report is one that involves death, a life-threatening event, new or prolonged hospitalization, disability or permanent damage, or a congenital anomaly. I will focus on the reports for all AE’s.
They also have a table that calculates PRRs by comparing reports for the Pfizer COVID-19 vaccine to reports for the Moderna vaccine and vice versa, again for all reports, serious reports only and non-serious reports. There were no remarkable findings in those tables, so I will not discuss them. [Edit: I forgot what Norman Fenton noted in his analysis: the overall proportion of reports with serious adverse events is 9.6% for Modern compared to 12.6% for Pfizer.] This isn’t that surprising since both vaccines are very similar and so should present relatively similar adverse events when compared to each other, and any differences are likely not large enough to be picked up by a PRR analysis. [Though the difference in the overall rate of serious adverse events, which are not specific to a particular type of event only how serious it is, was significant.]
The CDC seems to have calculated PRRs for every different type of adverse event reported for all the COVID vaccines examined - though it’s possible they only analyzed a subset. What seems clear is that, among the AEs they examined, the only ones included in the tables satisfy at least one of two conditions: a PRR value of at least 2 and a Chi-square value of at least 4 (Chi is the Greek letter χ and is pronounced like ‘kai’). When both conditions were met, they highlighted the adverse event in yellow, which appears to indicate a safety signal. There were no COVID vaccine AEs listed with fewer than 3 reported events, though for non-COVID vaccines there were many AEs listed that had only 1 or 2 reported since 2009. The CDC tables still include these and highlight them in yellow when the PRR is greater than 2 and the Chi-square value is great than 4, indicating these events are counted as safety signals.
WHAT SAFETY SIGNALS DID THE CDC FIND?
I’m going to divide this up by age groups and the Pfizer v. Moderna comparison. Let’s start with the 18+ group.
There are 772 AEs that appear on the list. Of these, 770 are marked in yellow and have PRR and Chi-square values that qualify them as safety signals. Some of these are new COVID-19 related codes, and we would expect those to trigger a signal since they didn’t exist in prior years to be reported by other vaccines. So if we take those off, we are left with 758 different types of non-COVID adverse events that showed safety signals.
I grouped these 758 safety signals into different categories. The figure below shows the total number of AEs reported for each of the major categories of safety signals:
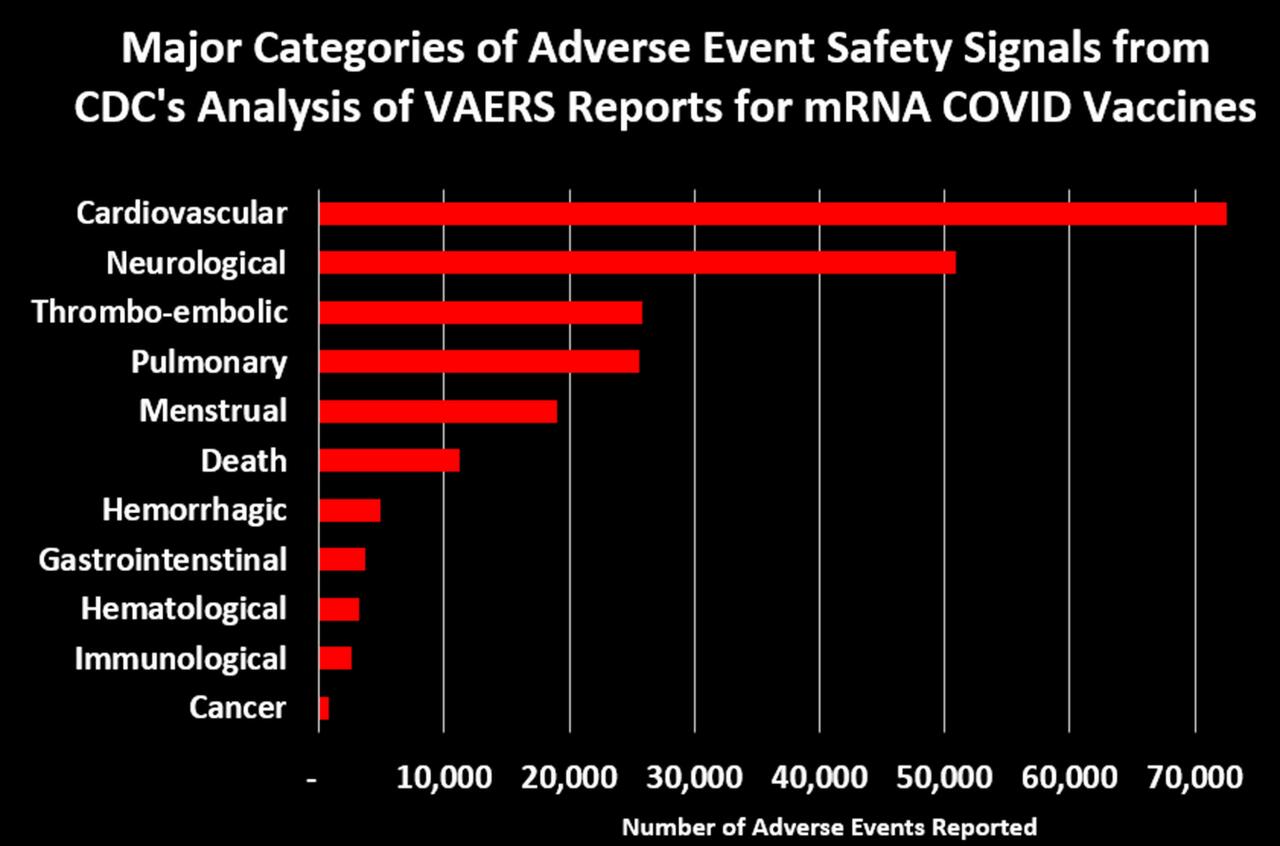
Let’s dig into some of these categories to look at what types of AEs generated the most number of reports:3
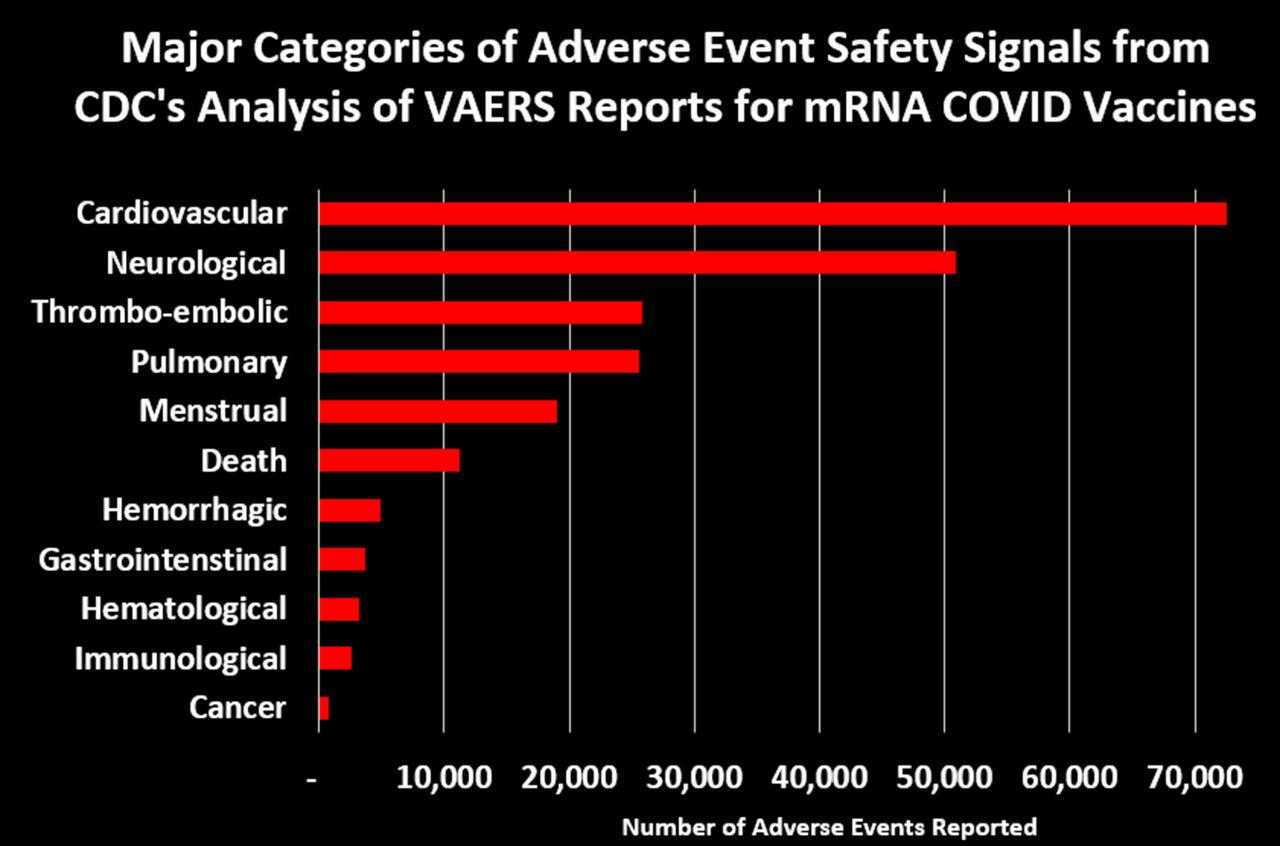
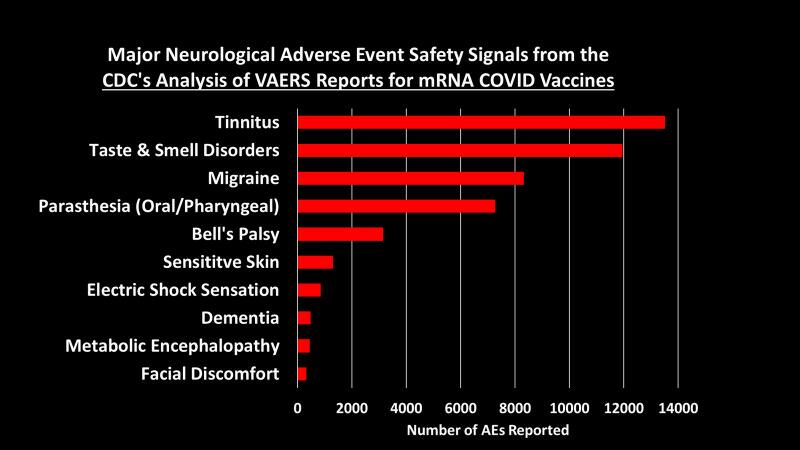
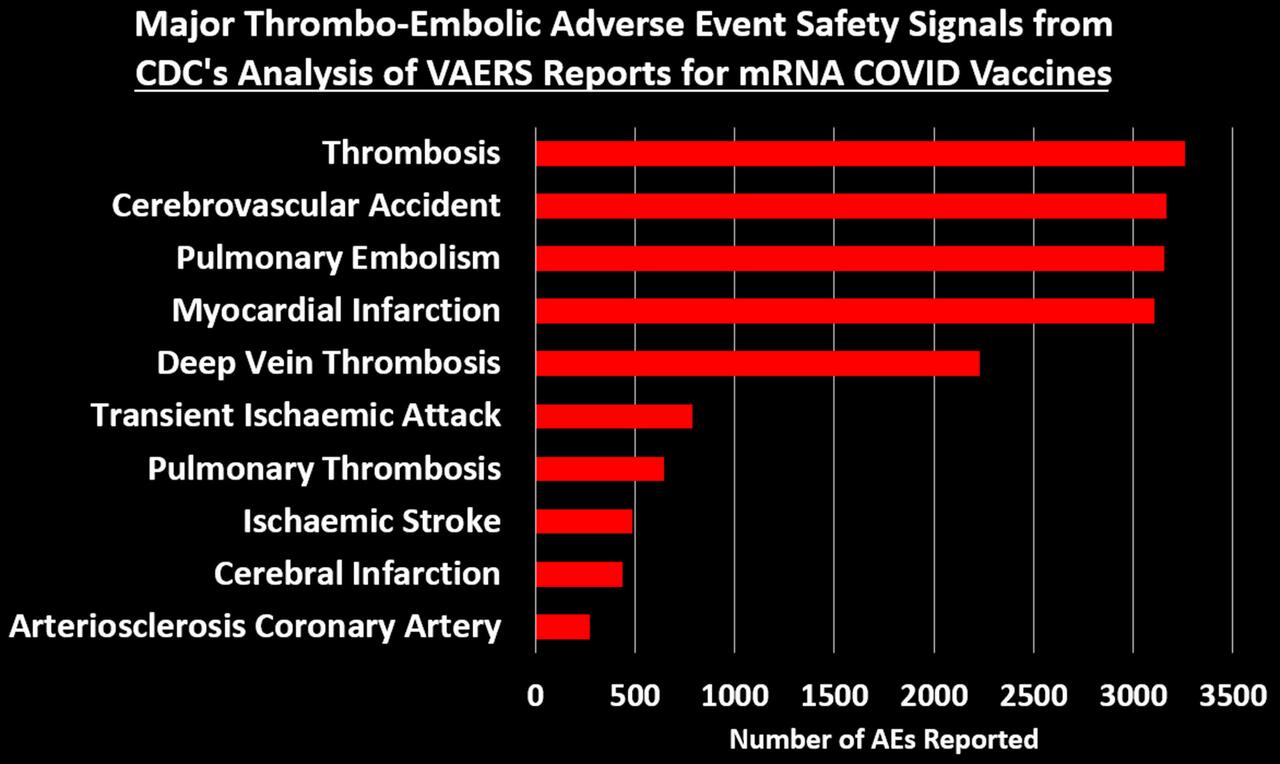
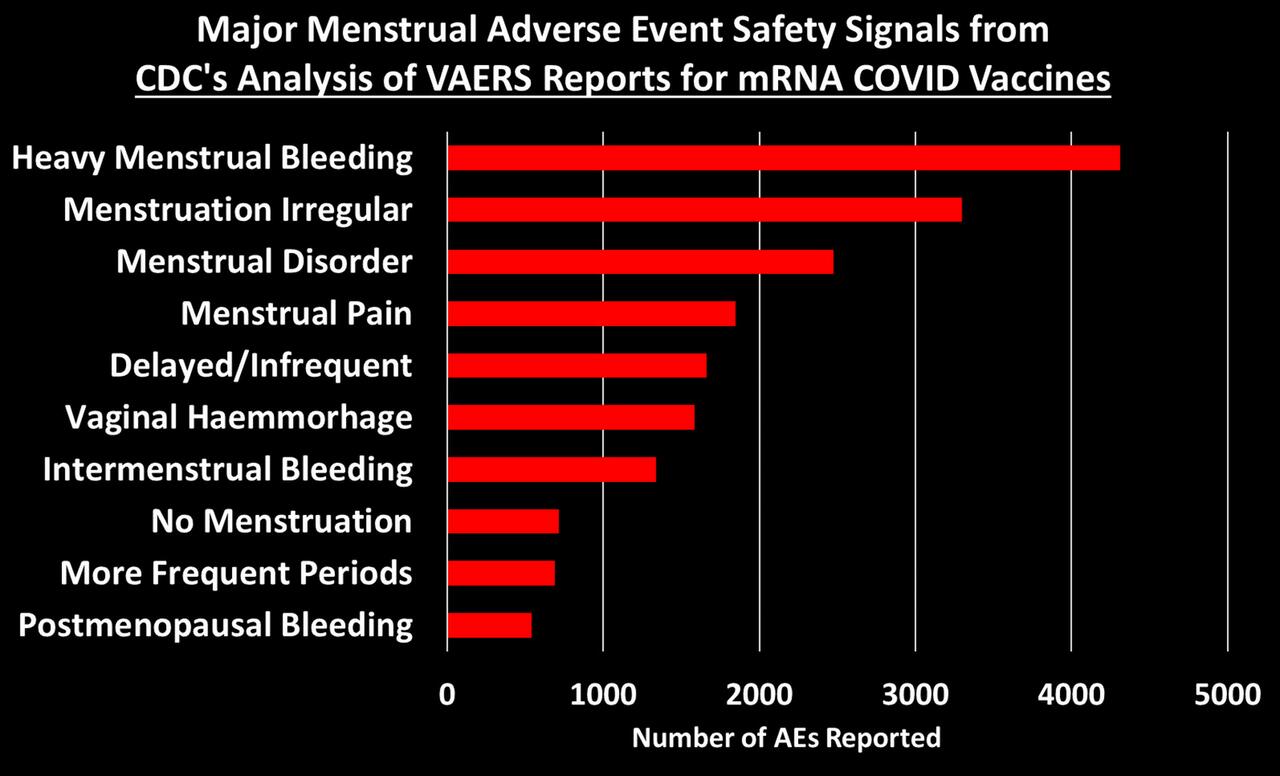
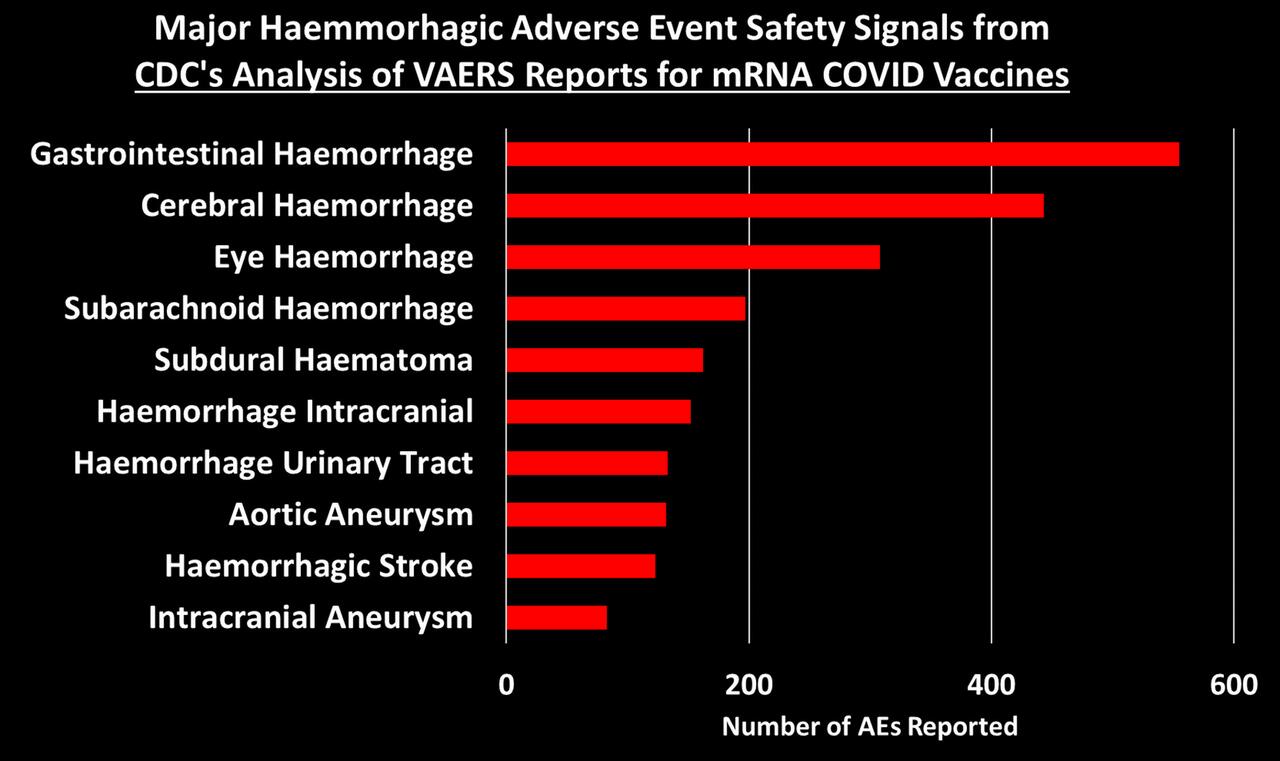

You can peruse the adverse events using the Excel tables provided by the CDC, which were posted by The Epoch Times and Children’s Health Defense at the links at the top of this post.
What about The Children?
If there is anything that looks remotely like a bright spot in all of this is that the list of safety signals for 12-17 and 5-11 year-olds is much shorter than for 18+. There are 96 AEs that qualify as a safety signal for the 12-17 group and 67 for the 5-11. When we take out the new COVID-era AEs, there are 92 safety signals for 12-17 year-olds and 65 for 5-11 year-olds. Here are the most alarming ones:
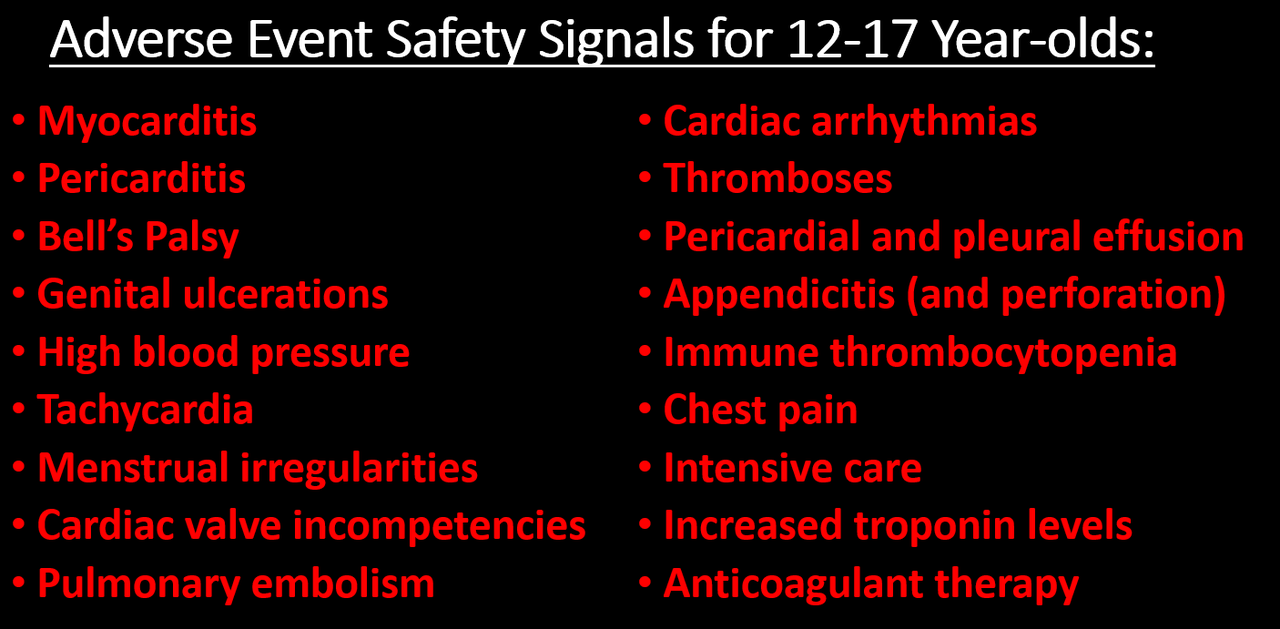

I don’t know why the list of AE’s is so much shorter for these age groups. It could be that the list of AE’s for other vaccines for these age groups is much shorter, so in a case where AEs have been reported for the mRNA COVID vaccines but not for other vaccines, it will not be counted as a safety signal by definition.
COMPARISONS TO MYOCARDITIS & PERICARDITIS
We are told that the existence of a safety signal doesn’t necessarily mean the AE is caused by the vaccine, and I accept that premise. But the current practice seems to be to ignore safety signals, dismiss them as noise without any evidence, and stall any investigation into them as long as possible. The precautionary principle, however, dictates we should presume that a safety signal indicates causality, until proven otherwise. Since, it has been acknowledged that the mRNA COVID vaccines can cause myocarditis and pericarditis (often referred to as myo-pericarditis), we can take those AEs as a kind of benchmark, and propose that, at minimum, any AE with a signal of equal or greater size should be considered potentially causal and investigated more thoroughly.4
After dropping the new COVID-era AEs, there are 503 AEs with PRRs larger than myocarditis (PRR=3.09) and 552 with PRRs larger than pericarditis (PRR=2.82).5 This means that 66.4% of the AEs had a bigger safety signal than myocarditis and 77.3% were larger than pericarditis. You can see what those were by use this Excel file provided by the CDC and sorting the 18+ tab by the 12/14-07/29 PRR column (Column E). Then just look at which AEs have PRRs larger than the ones for pericarditis and myocarditis.
For 12-17 year-olds, there is 1 safety signal larger than myocarditis (it’s ‘troponin increased’) and 14 safety signals larger than pericarditis (excluding myocarditis), which include: mitral valve incompetence, bell’s palsy, heavy menstrual bleeding, genital ulceration, vaccine breakthrough infection, and a range of indicators of cardiac abnormalities.
For 5-11 year-olds, the comparison to myo/pericarditis is less germane, as they seem to suffer less from this side effect. But we can still make the comparison: there are 7 safety signals larger than pericarditis, including bell’s palsy, left ventricular dysfunction, mitral valve incompetence, and ‘drug ineffective’ (presumably meaning they still got COVID). There are 16 safety signals larger than myocarditis (excluding pericarditis), which in addition to those listed above also include: pericardial effusion, diastolic blood pressure increase, tricuspid valve incompetence, and vitiligo. Sinus tachycardia (high heart rate), appendicitis, and menstrual disorder come in just below myocarditis.
Now if we think of a safety signal as having both strength and clarity, then the PRR can be thought of as an indicator of how strong the signal is, while the Chi-square is a measure of how clear or unambiguous the signal is, because it gives us a sense of how likely the signal is due to chance alone: the larger the Chi-square value, the less likely the signal is due to chance. A Chi-square of 4 means there is only a 5% chance the observed signal is due to chance. A Chi-square of 8 means there is only a 0.5% chance of it being due to chance.6
For the 18+ group, there are 57 AEs with a Chi-square larger than myocarditis (Chi-square=303.8) and 68 with a Chi-square larger than pericarditis (Chi-square=229.5). Again, you can see what these are by going the Excel file linked above and sorting on Column D.
For the 12-17 group, there are 4 AEs with a larger Chi-square than myocarditis (Chi-square=681.5) and 6 larger than pericarditis (Chi-square=175.4).
For the 5-11 group, there are 22 AEs with a Chi-square larger than myocarditis (Chi-square=30.42) and 34 AEs with a Chi-square larger than pericarditis (Chi-square=18.86).
RESPONDING TO OBJECTIONS
Let’s dispense with some of the criticisms used to dismiss VAERS data, which will undoubtedly be raised if you try to bring the CDC’s analysis to people’s attention.
- Objection: Anybody can report to VAERS. The reports are unreliable. Anti-vaxxers made lots of fraudulent reports. Nobody was aware of VAERS in the past, but now they are. So many people were afraid of the vaccine so they blamed all their health problems on it. Health workers were required by law to report certain adverse events, like deaths and anaphylaxis. Etc. Etc.
All of these objections ultimately rely on the notion that VAERS reports for COVID-19 vaccines have been artificially inflated over previous years for one reason or another. The thing of it is, though, that the CDC has a method for distinguishing between artificial inflation and real signal. The idea is simple: if adverse events are artificially inflated, they should be artificially inflated to the same degree. Meaning, the PRRs for all of these safety signals should be about the same. But even a casual glance at the PRRs in the Excel file show they vary widely, from as low at 2 to as high as 105 for vaccine breakthrough infection or 74 for cerebral thrombosis. This method does not on the number of reports, but the rate of reporting for certain events out of all events reported. If anything, this method would tend to hide safety signals in a situation where a new vaccine generates a very large number of reports.
The CDC has even done us the favor of calculating upper and lower confidence intervals, meaning that we can be at least 95% confident that two PRRs are truly different if their confidence intervals don’t overlap. So for example the lower confidence interval for pulmonary thrombosis is 19.7, which is higher than the upper confidence interval for 543 other signals. Artificially inflated reporting cannot explain why so many different adverse events have large PRRs that are statistically distinct from one another.
- Objection: The safety signals are due to the huge number of COVID vaccines given out. Never before have we given out so many vaccine doses. By the end of July, the US had administered something like 600 million vaccine doses to people aged 18+. But the CDC analysis compares VAERS reports for these doses to all doses for all other vaccines for this age group since Jan. 1, 2009. But from 2015-2020 there were over 100 million flu doses administered annually to this age group alone. In previous work, I estimated 538 million doses of flu given to people 18+ from July 2015-June 2020. The number of flu and other non-COVID vaccines for this age group administered from Jan 1., 2009 through July 29, 2022 must be well over double this number, meaning VAERS reports for COVID vaccines are being compared to reports for at least double the number of doses for other vaccines. In addition to this, as already noted, the PRR methodology does not depend, strictly speaking, on the number of doses, but rather the rate of reporting of a specific AE out of all AEs for that vaccine.
- Objection: the vaccines are mainly being given to older people who tend to have health problems, whereas other vaccines are given to younger people. This objection is dealt with, since the analyses are stratified by age groups. It might be still be somewhat valid for the 18+ group, except that in the safety signal analysis I did in the fall of 2021, I stratified by smaller age bands and still found safety signals. In any case, this objection is not enough to dismiss the safety signal analysis out of hand, but rather calls for better and more refined research.
- Objection: The VAERS data is not verified and cannot be trusted. I’ll be the first person to agree that VAERS is not high quality data, but if it is completely untrustworthy, then how is it that the CDC uses these data to publish in the best medical journals such as JAMA and The Lancet? If the data were worthless, then these journals shouldn’t accept these papers. In that JAMA paper, they reported that 80% of the myocarditis reports met their definition of myocarditis and were included in the analysis. Many other reports simply needed more details for validation. Furthermore, the CDC has the ability and budget to follow-up on every report VAERS receives to get more details and even medical records to verify the report.
So if myocarditis shows a clear signal in the CDC’s analysis, and 80% of those reports were apparently high quality enough to be included in a paper published in one of the world’s top medical journals, how is it possible that all the rest of the reports are junk? That all of the other safety signals are meaningless? Answer: it isn’t.
And since we’re on the topic of safety signals that turned out to be real, it’s instructive to find appendicitis turn up as a safety signal in all 3 age groups, since a study published in NEJM based on medical records of over a million adult Israelis found an increased risk of appendicitis in the 42 days following Pfizer vaccination (but not following a positive SARS-CoV-2 PCR test). That study also found an increase in lymphadenopathy (swollen lymph nodes) after vaccination, but not after positive COVID test. Lymphadenopathy was another safety signal.
- And that brings us to our last objection to be dispensed with: all of these AEs were due to COVID. There was an epidemic and so people were falling ill due to COVID and having all of these problems that were then blamed on the vaccine. Well to begin with, as we just saw, at least two of them (appendicitis and lymphadenopathy) do not appear to have increased risk ratios following a positive SARS-CoV-2 test, and we know that the mRNA vaccines increase risk of myo/pericarditis independent of infections. So how can we assume the rest of these are and dismiss them with the wave of a hand? We can’t. At minimum, they need further investigation. Furthermore, in the safety signal analysis I did in 2021, I dropped all VAERS reports where any sign of a SARS-CoV-2 exposure or infection was indicated on the report, and I still found large, significant safety signals.
PUTTING IT ALL INTO PERSPECTIVE
The Epoch Times article quotes my esteemed colleague and friend, Norman Fenton, Professor of Risk Management and an world renowned expert in Bayesian statistical analysis: “from a Bayesian perspective, the probability that the true rate of the AE of the COVID-19 vaccines is not higher than that of the non-COVID-19 vaccines is essentially zero…. The onus is on the regulators to come up with some other causal explanation for this difference if they wish to claim that the probability a COVID vaccine AE results in death is not significantly higher than that of other vaccines.” (See his post on the CDC analysis here.) The same is true for all the safety signals they found.
The CDC’s VAERS SOP analysis document lists 18 Adverse Events of Special Interest says they are going to pay close attention to. In their 2021 JAMA paper (and similar presentations to ACIP), the researchers responsible for analyzing the millions of medical records in the CDC’s Vaccine Safety Datalink (VSD) using the ‘Rapid Cycle Analysis’ only studied 23 outcomes. A Similar analysis in NEJM from Israeli researchers focused on only 25 outcomes. Compare this to over 700 safety signals found by the CDC when they finally decided to look—and that’s not even counting all the adverse events that have never been reported for other vaccines so cannot ever show a safety signal by definition. How can the CDC say that these safety signals are meaningless if almost none of them have been studied any further? And yet we are assured that these vaccines have undergone the most intensive safety monitoring effort in history. It’s complete and utter hogwash!
* * *
Josh Guetzkow is a senior lecturer at The Hebrew University of Jerusalem. Subscribe to his Substack here.
1) To be precise, the 'adverse events' are for 'preferred terms' (PTs) which is a type/level of classification used in the Medical Dictionary for Regulatory Activities (MedDRA), which is the classification system used by VAERS and in other pharmacovigilance systems and clinical research for coding reported adverse events. Not all preferred terms are a symptom or adverse event per se. Some refer to a specific diagnostic test that was done or a treatment that was given.
2) It's not entirely clear how they divided these up, since there are clearly AEs that should be considered serious that don't show up in the serious Excel table — though maybe they don’t come up simply because they are looking within serious reports. I believe that they just filtered the reports to include only serious reports or non-serious reports, then did the safety signal analysis on all the AE's coded in those reports. The reason I think this is that I used the MedAlerts Wayback Machine, selected just the serious COVID-19 vaccine reports, and the numbers of total reports was very close to the one in the table provided by the CDC (MedAlerts actually had a bit less). The files obtained by the Epoch Times do not include much in the way of a description as to how the analyses were done, so I had to infer some details, which might be incorrect. I will try to note when I am drawing an inference about how the analysis was done.
3) Generally speaking, these figures show the top ten AEs in each category. In some cases I combined AEs that indicated the same thing, such as combining ‘heart rate irregular’ with ‘arrythmia.’ [UPDATE: Note that the charts of all categories, cardiac and thrombo-embolic events were updated on Jan 7, 2023. The reason is that I had previously categorized acute myocardial infarction as a cardiac issue and myocardial infarction as thrombo-embolic. To be consistent, I have now combined myocardial infarction and acute myocardial infarction into one AE category in the thrombo-embolic events (which made the total AEs reported for that category larger than for pulmonary ones) and then added a different cardiac AE to the cardiovascular AE category, ventricular extrasystoles, AKA premature ventricular contraction (PVC), which dependent on frequency and the presence of other cardiomyopathies is associated with sudden cardiac arrest.]
4) Note that using the myo-pericarditis signal as a yardstick doesn’t mean that these are the only signals that matter. To give one example, anaphylactic reactions don’t even show up in the list of safety signals, even though that was one of the very first risk of the vaccine that became apparent from day one of the vaccine rollout.
One potential objection to this benchmark is that it is too low of a bar, since myo-pericarditis appears to disproportionately affect younger men and so a proper safety signal should be stratified by age and gender then compared with myocarditis similarly stratified. I agree, and it is the CDC’s job to do that. But the fact is that any adverse reaction might disproportionately affect some subgroup of people, in which case the safety signal for that group would be similarly faint or diluted when we look at everyone together. So objection overruled.
5) In their Standard Operation Procedures document, the CDC said they would combine these and related codes together to assess a safety signal, but never mind – at least they finally got around to doing something.
6) In this context, the Chi-square is largely driven by the sheer number of adverse events: the more adverse events reported, including for the comparator vaccine, the larger the Chi-square. For example, the PRR for pericarditis and subdural haematoma is the same (2.82), but there were 1,701 incidents of pericarditis reported for mRNA COVID vaccines versus 221for the comparator vaccines, with Chi-square of 229.5. For subdural haematoma, these numbers are 162 verus 21, for a Chi-square of 21.2.
The Question That Terrifies Branch Covidians: Why Has Covid Spared Africa Where Only 6% Are Vaxxed?
STORY AT-A-GLANCE
- There are clear contradictions between the World Health Organization’s directives regarding the need for COVID shots in Africa and the actual situation on the ground
- The WHO is still calling on all countries to get the COVID jab into at least 70% of their populations, and warns that developing countries are at grave risk due to low jab rates. Meanwhile, Africa, where less than 6% of the population is jabbed, has fared far better than countries with high injection rates. A large-scale survey in Uganda also shows COVID is no longer a clinical issue
- Variants have also gotten milder (less pathogenic) with each iteration, yet the WHO warns that new variants may create “large waves of serious disease and death in populations with low vaccination coverage”
- The explanation for the disconnect between the WHO’s priorities and what’s happening in Africa can be explained when you look at the focus of the WHO’s Catastrophic Contagion exercise. It focused on getting African leadership trained in following the pandemic script. The WHO needs additional pandemics in order to justify its pandemic treaty, which will give it sole power to dictate countermeasures, and it needs to eliminate the African control group, which shows the COVID “vaccines” do more harm than good
- The WHO also has every intention of implementing climate lockdowns once it has the power to do so. To that aim, the WHO’s director of Environment and Health has suggested combining health and climate issues into one
In the video above, John Campbell, Ph.D., a retired nurse educator, compares the contradictions between the World Health Organization’s directives regarding the need for COVID shots in Africa and the actual situation on the ground.
As of December 12, 2022, the WHO was still calling on all countries to get the COVID jab into at least 70% of their populations.1 Its original deadline for meeting this 70% threshold was mid-2022, but by June 2022, only 58 of 194 member states had reached this target.2
According to the WHO, jab supplies, technical support and financial support were lacking during the early days of the injection campaign but, now, those obstacles have been resolved. As a result, all countries now have the ability to meet the global target of 70%.
Low Jab Rates Threaten Low-Income Countries, WHO Claims
The “overarching challenge” right now is the administration of the shots, actually “getting shots into arms.”3 To address that, the WHO suggests integrating COVID-19 injection services “with other immunization services and alongside other health and social interventions.” This, they say, will maximize impact and “build long-term capacity.”
The WHO also stresses that “As people’s risk perception of the virus wanes, careful risk communication and community engagement plans need to be adapted to enhance demand for vaccination.” To ensure low-income countries get onboard to meet the 70% target, the WHO also launched The COVID-19 Vaccine Delivery Partnership in January 2022.
This is an international effort “to intensify country readiness and delivery support” in 34 countries with low COVID jab uptake. Partners include UNICEF, Gavi and the World Bank. According to the WHO:4
“Despite incremental success since its launch in January 2022, low and lower-middle income countries are facing difficulties to get a step change in vaccination rates.
This represents a serious threat to the fragile economic recovery, including due to the risk of new variants creating large waves of serious disease and death in populations with low vaccination coverage.
It also means accelerating the delivery of other COVID-19 tools and treatments is a crucial priority to help the world build up multiple layers of protection against the virus. Concerted and urgent action from countries, international partners and agencies, along with G20 Finance Ministers is required to increase vaccination levels and expedite access.”
In short, the WHO is really concerned that countries with low COVID jab rates will suffer lest they meet or exceed the target goal of jabbing 70% of their populations. But what is that concern based on? Certainly not the real world.
WHO’s Statements Contradict Real-World Situations
The statements made by the WHO contradict a number of real-world situations. For starters, while developed nations with high jab rates struggled with COVID-19 throughout much of 2021 and 2022, Africa avoided this fate, despite its single-digit jab rate.
Scientists are said to be “mystified” as to how Africa fared so well, completely ignoring data showing that the more COVID shots you get, the higher your risk of contracting COVID and ending up in the hospital.
Over the past year, researchers have been warning that the COVID jabs appear to be dysregulating and actually destroying people’s immune systems, leaving them vulnerable not only to COVID but also other infections.5 It stands to reason, then, that Africa with its low injection rate would not be burdened with COVID cases brought on by dysfunctional immune systems.
Secondly, variants have gotten milder (less pathogenic) with each iteration, albeit more infectious (i.e., they spread easier). So why is the WHO worried about “the risk of new variants creating large waves of serious disease and death in populations with low vaccination coverage”? What is that “risk” based on?
And, since COVID infection keeps getting milder, and has had a lethality on par with or lower than influenza6,7,8,9,10 ever since mid-2020 at the latest, why is it still a “crucial priority” to accelerate delivery of COVID treatments?
As a reminder, according to a September 2, 2020 study in Annals of Internal Medicine, the overall noninstitutionalized infection fatality ratio for COVID was a mere 0.26%. Below 40 years of age, the infection fatality ratio was just 0.01%. Meanwhile, the estimated infection fatality rate for seasonal influenza is 0.8%.11
Report From Uganda
Campbell goes on to cite a large-scale survey by a community health partner in Uganda, which surveyed doctors, nurses and medical officers across the country, and “basically, they don’t see any COVID anymore,” he says.
They’re not getting the jab and they’re not getting tested for COVID either. There’s no need, because no one is getting sick with COVID — at least not to the point they need medical attention.
The Ugandan government has even stopped publishing COVID guidelines. From their perspective, the pandemic is over. The same sentiment appears common in other African countries as well. Given the situation on the ground, is it really a pressing need to jab 30 million people in Uganda against a disease they’re not getting sick from?
What Uganda does need is malaria treatments, mosquito nets, clean drinking water and antibiotics. “That is what the priorities on the ground seem to be,” Campbell says. So, what’s with the apparent disconnect between the WHO’s priorities and what’s actually happening in areas with low COVID jab rates? The WHO’s Catastrophic Contagion exercise12,13 clues us in.
The Disconnect Reveals the WHO’s True Intentions
October 23, 2022, the WHO, Bill Gates and Johns Hopkins cohosted a global challenge exercise dubbed “Catastrophic Contagion,”14,15 involving the outbreak of a novel pathogen called “severe epidemic enterovirus respiratory syndrome 2025” (SEERS-25).
Tellingly, this tabletop exercise was focused on getting African leadership involved and trained in following the pandemic script. Participants included 10 current and former health ministers and senior public health officials from Senegal, Rwanda, Nigeria, Angola and Liberia. (Representatives from Singapore, India and Germany, as well as Gates himself, were also in attendance.)
African nations just so happened to go “off script” more often than others during the COVID pandemic and didn’t follow in the footsteps of developed nations when it came to pushing the jabs. As a result, vaccine makers now face the problem of having a huge control group, as the COVID jab uptake on the African continent was only 6%.16
They cannot reasonably explain how or why Africa ended up faring so better than developed nations with high COVID jab rates in terms of COVID-19 infections and related deaths.17
The WHO’s pandemic treaty is the gateway to a global, top-down totalitarian regime. But to secure that power, they will need more pandemics.
The WHO desperately needs to get rid of this control group, so they’re enlisting and training African leaders how to push for widespread vaccination using the WHO’s talking points. This, I believe, is the only reason the WHO is still speaking about COVID-19 in catastrophic terms.
The WHO Needs Additional Pandemics to Secure Its Power Grab
At this point, it’s quite clear that “biosecurity” is the chosen means by which the globalist cabal intends to usher in its one world government. The WHO is working on securing sole power over pandemic response globally through its international pandemic treaty which, if implemented, will eradicate the sovereignty of member nations.
The WHO’s pandemic treaty is basically the gateway to a global, top-down totalitarian regime. But to secure that power, they will need more pandemics. COVID-19 alone was not enough to get everyone onboard with a centralized pandemic response unit, and they probably knew that from the start.
So, the reason we can be sure there will be additional pandemics, whether manufactured using fear and hype alone or an actual bioweapon created for this very purpose, is because the takeover plan, aka The Great Reset, is based on the premise that we need global biosecurity surveillance and a centralized response.
The Biden-McCarthy-Schumer era has begun, which means your wealth or retirement is in jeopardy. The smart money is getting physical precious metals sent straight to their door and rolling over their retirement to self-directed IRAs.
Biosecurity, in turn, is the justification for an international vaccine passport, which the G20 just signed on to, and that passport will also be your digital identification. That digital ID, then, will be tied to your social credit score, personal carbon footprint tracker, medical records, educational records, work records, social media presence, purchase records, your bank accounts and a programmable central bank digital currency (CBDC).
Once all these pieces are fully connected, you’ll be in a digital prison, and the ruling cabal — whether officially a one world government by then or not — will have total control over your life from cradle to grave.
The WHO’s pandemic treaty is what sets this chain of events off, as it will have the power to implement vaccine passports globally once the treaty is signed. The WHO will also have the power to mandate vaccines, standardize medical care and issue travel restrictions.
This treaty will likely pass this year, which means the WHO will either need to ramp up the COVID narrative again, or switch to another pandemic in order to justify these kinds of actions.
With 90% of ingredients in pharmaceuticals coming from Communist China, the risk of a medical supply chain crisis has never been greater. Secure five types of antibiotics to have ready when you need. Use promo code “Rucker10” for $10 off.
The Pandemic Treaty Is the Death Knell to Freedom Worldwide
It’s important to realize that the WHO’s pandemic treaty will radically alter the global power structure and strip you of some of your most basic rights and freedoms. It’s a direct attack on the sovereignty of its member states, as well as a direct attack on your bodily autonomy.
Once signed, all member nations will be subject to the WHO’s dictates. If the WHO says every person on the planet needs to have a vaccine passport and digital identity to ensure vaccination compliance, then that’s what every country will be forced to implement, even if the people have rejected such plans using local democratic processes.
There’s also reason to suspect the WHO intends to extend its sovereign leadership into the health care systems of every nation, eventually implementing a universal or “socialist-like” health care system as part of The Great Reset. WHO Director-General Tedros has previously stated that his “central priority” as director-general of the WHO is to push the world toward universal health coverage.18
Prediction: Climate Lockdowns Are Next
Considering the WHO changed its definition of “pandemic” to “a worldwide epidemic of a disease,”19 without the original specificity of severe illness that causes high morbidity,20,21 just about anything could be made to fit the pandemic criterion. This means that once they’re in power, they won’t need to rely exclusively on pathogenic threats.
They could also declare a global pandemic for a noninfectious threat, like global warming, for example. Such a declaration would then allow the WHO to circumvent laws that are in place to preserve our freedom, and allow for the implementation of tyrannical measures such as lockdowns and travel restrictions.
Indeed, the notion of “climate lockdowns” has already been publicly flouted on multiple occasions.22 As reported by The Pulse:23
“Climate lockdowns and other restrictions will be framed as saving the people of the world from themselves. Who would ever disagree with such measures when it is framed under the guise of good will?
Like we saw with COVID mandates, if climate mandates ever take place they will be promoted as an extremely noble and necessary action. Those who disagree and present evidence that such actions are not useful or impactful, and instead cause more harm, will most likely be silenced, censored and ridiculed …
What would a climate lockdown look like? Well, if such an initiative were to take place, governments would limit or ban the consumption of many foods. They would ban or limit private-vehicle use, or limit the distance one can travel in a gas powered car or perhaps even by plane.
Working from home could eventually become the permanent norm if special carbon taxes are put in place. Such taxes could be imposed on companies, limiting driving or air miles, and extend to individual employees … Schools, especially those heavily influenced by teachers’ unions, could impose permanent online-only days.”
Officials Around the World Have Suggested Climate Lockdowns
As noted by The Pulse, a number of officials around the world have voiced support for climate lockdowns, completely ignoring the devastating effects the COVID lockdowns have already had. This just goes to show lockdowns were never about public health and never will be.
Among the climate lockdown enthusiasts we have Germany’s health minister Karl Lauterbach, who in December 2020 proclaimed that addressing climate change would require restrictions on personal freedom, similar to those implemented to “flatten the curve” of COVID.24
British economics professor Mariana Mazzucato is another advocate for climate lockdowns, who in September 2020 warned that “In the near future, the world may need to resort to lockdowns again — this time to tackle a climate emergency.”25
We also have the statements of Bill Gates26 and the Red Cross,27 both of which in 2020 claimed that climate change poses a greater threat to mankind than COVID, and must be confronted with the same urgency and resolve. The World Economic Forum (WEF), the United Nations and the WHO have also published articles stating their intent to “fight climate change” by shutting down society.28
Notably, in “How to Fight the Next Threat to Our World: Air Pollution,” published by the WEF29 and co-written by the director of WHO’s Environment and Health Department, it’s suggested that health and climate issues be combined into one. As noted in that article:
“We can confront these crises more effectively and fairly if we address them as one — and foster support across all sectors of the economy … COVID-19 has proven humanity’s inbuilt ability to rise up and act to protect the health of our most vulnerable people. We need to do the same with air pollution.”
Recall, as I mentioned above, if the WHO has sole power over global health, combining health and climate issues will automatically give the WHO the de facto power to issue climate lockdowns. Some claim climate lockdowns have already begun,30 with the random shutting off of people’s power even though there’s no actual outage — sort of slow-walking people into accepting that the lights won’t always turn on.
That the WHO will jump at the opportunity to implement climate lockdowns can also be seen in the WHO Manifesto for a Healthy Recovery From COVID-19, which states:31
“The ‘lockdown’ measures that have been necessary to control the spread of COVID-19 have slowed economic activity, and disrupted lives — but have also given some glimpses of a possible brighter future.
In some places, pollution levels have dropped to such an extent that people have breathed clean air, or have seen blue skies and clear waters, or have been able to walk and cycle safely with their children — for the first times in their lives.
The use of digital technology has accelerated new ways of working and connecting with each other, from reducing time spent commuting, to more flexible ways of studying, to carrying out medical consultations remotely, to spending more time with our families.
Opinion polls from around the world show that people want to protect the environment, and preserve the positives that have emerged from the crisis, as we recover …
Decisions made in the coming months can either “lock in” economic development patterns that will do permanent and escalating damage to the ecological systems that sustain all human health and livelihoods, or, if wisely taken, can promote a healthier, fairer, and greener world.”
This manifesto also lays out many other aspects of The Great Reset agenda, including smart cities, travel restrictions, new food systems, a complete transition to green energy and more. But again, the thing that will really facilitate all of these changes is to have a centralized powerbase, and that is the WHO.
What Can You Do?
Stopping the WHO pandemic treaty will be difficult, as the World Health Assembly may or may not even accept public comment before making a decision. Your best bet right now is to sign up for the World Council for Health’s (WCH) newsletter.
The last time the World Health Assembly met to discuss the treaty, the WCH issued links and instructions on how to submit your comment. You can subscribe at the bottom of this page, or on the WCH’s home page. I and the CHD will also share details if they become available, so subscribing to our newsletters can give you a heads-up as well.
In the absence of instructions, you could reach out to your respective delegation and request that they oppose the treaty. A list of U.S. delegates can be found in James Roguski’s Substack article, “Speaking Truth to Power.”
For contact information for other nations’ delegates, I would suggest contacting the regional office and ask for a list (see “Regions” in the blue section at the bottom of the World Health Assembly’s webpage).
- 1 WHO COVID-19 Vaccines
- 2, 3, 4 WHO Vaccine Equity
- 5 medRxiv May 6, 2021
- 6 The Mercury News May 20, 2020 (Archived)
- 7, 11 Annals of Internal Medicine September 2, 2020 DOI: 10.7326/M20-5352
- 8 Breitbart May 7, 2020
- 9 Scott Atlas US Senate Testimony May 6, 2020 (PDF)
- 10 John Ioannidis US Senate Testimony May 6, 2020 (PDF)
- 12, 14 Catastrophic Contagion
- 13, 15 Catastrophic Contagion Videos
- 16 First Post November 19, 2021
- 17 Yahoo News November 19, 2021
- 18 National Review June 14, 2017
- 19 Wayback Machine, WHO Pandemic Preparedness captured September 2, 2009 (PDF)
- 20 The BMJ 2010;340:c2912
- 21 Wayback Machine, WHO Pandemic Preparedness captured May 1, 2009 (PDF)
- 22 The Hill February 2, 2022
- 23 The Pulse November 25, 2022
- 24 Welt December 27, 2020
- 25 Project-syndicate.org September 22, 2020
- 26 Gates Notes August 4, 2020
- 27 Aljazeera November 17, 2020
- 28, 30 Center for the Preservation of Humanity September 8, 2022
- 29 WEF September 7, 2022
- 31 WHO Manifesto for a Healthy Recovery From COVID-19
COVID Vaccines Are "Obviously Dangerous" And Should Be Halted Immediately, Say Senior Swedish Doctors
SATURDAY, JAN 14, 2023 - 06:10 AM
Authored by Dr. Johan Eddebo via The Daily Sceptic,,
There follows a public statement by a group of five senior Swedish doctors who, in collaboration with Dr. Johan Eddebo, a researcher in digitalisation and human rights, are raising the alert about the Covid vaccines, which they describe as “obviously dangerous”. They say there should be an “immediate halt” to the mass vaccination pending “thorough investigations” of the true incidence and severity of adverse effects.
The true character and scope of the harm caused by the unprecedented mass vaccinations for COVID-19 is just now beginning to become clear. Leading scientific journals have finally begun publishing data corroborating what the underground research community has observed over the last two years, especially in relation to complex problems of immune suppression.
Truly concerning numbers pertaining to both births and mortality are also emerging.
At this moment in time, a new, allegedly super-infectious Omicron variant is all over the headlines. A sub-variant of XXB, this strain is said to possess immune escape capabilities of precisely the type that some independent researchers predicted would follow on the heels of the mass vaccinations’ narrow antigenic fixation.
The WHO maintains that worldwide, 10,000 people still die due to Covid every single day, an implausible death toll more than ten times that of an average flu. It reiterates the urgent need for vaccinations, especially in light of China’s reopening and allegedly falsified data on mortality and infections.
The EU has even called an emergency summit in light of the purported Chinese “Covid chaos” that “calls to mind how everything began in Wuhan, three years ago”.
In Sweden, the Minister for Health and Social Affairs has said he cannot rule out new restrictions, and states that everyone must take “their three doses”, since “only” 85% of the population is ‘fully inoculated’.
That such an extensive vaccine coverage has not yielded better results after nearly two years is a remarkable fact. Even more so in light of some individuals receiving four or more repeated exposures to the same vaccine antigen, yet still contracting the disease they are supposedly immunised against.
At the same time, even more ominous warning signs abound.
One such warning sign is the fact that average mortality in many Western states is still at a remarkably high level, in spite of the direct effects of the coronavirus being marginal for more than a year. Data from EuroMOMO indicate a marked excess mortality in the EU for all of 2022, and the German Bureau of Statistics reports that the country’s mortality in October was more than 19% over the median value of the preceding years.
Is this due to Covid, as the WHO’s ’10 000 per day’ figure would seem to indicate?
Blame is placed at the feet of ‘Long Covid‘ as well as the regular acute infections, but according to the EuroMOMO and Our World in Data stats, the bulk of the excess deaths in Europe during 2022 are actually not due to clinically manifest coronavirus infections.
Moreover, we shouldn’t see continued excess deaths from a respiratory virus of this kind after three years of global exposure due to the inevitable consolidation of natural immunity.
If such a situation persists, the hypothetical connection to a vaccine-related immunity suppression that just now has come into focus becomes pertinent to investigate in detail.
If, as has been argued, the vaccinations, and especially the boosters, alter the immune profile of recipients such that Covid infections get ‘tolerated’ by the immune system, it’s possible that vaccinated individuals will tend towards a situation of long-term, repeat infections that do not get cleared, and do not present with obvious symptoms, while still promoting systemic damage.
The literature now indicates an extensive substitution in the vaccinated of virus-neutralising antibodies for non-inflammatory ones, a ‘class switch’ from antibodies that work towards clearing the virus from our system, to a category of antibodies whose purpose is to desensitise us to irritants and allergens.
The net effect is that the inflammatory response to Covid infection gets down-regulated (reduced). This means that full-blown infections will present with milder symptoms, and that they won’t get cleared as effectively (partly since fever and inflammation are essential to your body getting rid of a pathogen).
That these developments alone aren’t cause for an immediate halt to the mass vaccinations, as well as thorough investigations, is astonishing.
There is of course another, and more well-known, potential partial explanation of the surprising excess mortality. We have indications of clotting disorders connected to the Covid vaccines, evident in a new major Nordic study, while repeated studies evidence a clear correlation between heart disease and Covid vaccination (see Le Vu et al., Karlstad et al. and Patone et al.).
A newly published Thai study moreover indicated that almost a third of the vaccinated youth enrolled exhibited cardiovascular manifestations, and a yet unpublished Swiss study suggests that as many as 3% of everyone vaccinated manifest heart muscle damage.
And as stated above, we also see signals pertaining to fertility disturbances connected to the Covid vaccines.
An Israeli study shows impaired motility and sperm concentrations after both Pfizer and Moderna vaccination. The safety committee of the European Medicines Agency has also affirmed that the vaccines may cause menstrual disturbances, and Pfizer’s own studies indicate that the lipid nanoparticles of the mRNA-vaccines cluster in the reproductive organs.
The hypothesis that COVID-19 vaccinations influence fertility is supported by a significant and unprecedented decline in the Swedish birth rate during the first months of 2022. According to Swedish demographers, the decline is ”surprising”.
There are similar data from many other Western countries, and to continue the mass vaccinations for low-risk groups such as children or pregnant women is utterly irresponsible – especially since the vaccinations do little or nothing to stop the spread as was initially promised, and is often still falsely maintained.
One hopes that the hypothesis of a decline in birth rates due to the vaccinations can be falsified through a thorough and independent investigation as soon as possible. The numbers are truly worrying.
Yet the fact that Pfizer’s data pertaining to fertility disturbances had been hidden away and needed to be discovered through a FOIA request is typical for the entire situation.
There’s almost no independent public debate on these issues, and critical perspectives are actively suppressed by the major digital platforms.
Public watchdogs such as the European Medicines Agency are funded by the pharmaceutical industry and often base their recommendations on Big Pharma’s in-house studies. The independence of our scientific and academic institutions is threatened, and we see a confluence between scientific research, private corporate interests and political and ideological objectives on every level.
To place a digital filter of censorship on top of all of this, where proprietary algorithms micromanage the flow of information and the public debate in accordance with the intentions of their owners, in practice means to abolish the open democratic society and independent scientific research.
Recent disclosures also show that the digital platforms have actively worked towards suppressing critical perspectives on the Covid policies and the mass vaccinations. Twitter has for this purpose developed clandestine censorship strategies and employed so-called ‘shadowbanning’ with the effect of an almost undetectable suppression of the visibility of posts and accounts connected to undesirable perspectives and analyses. Facebook took down more than seven million posts to influence the debate on Covid only during the second quarter of 2020. YouTube has banned publishing of video material that contains critical perspectives on the Covid vaccinations. Such content is designated ‘misinformation’ and ‘disinformation’ whether or not it is supported by relevant data.
These kinds of measures have very serious consequences. Digitalisation’s centralised control of the flow of information doesn’t just affect policy on the local and regional level, but also influences the way in which scientific and journalistic work can be designed and carried out. It creates structures that immediately repress heterodox views and silences critical voices through fear and indirect persecution.
Public trust in our common institutions will inevitably be eroded by this development.
The open society now desperately needs a renaissance. The democratic and scientific discourses must be rebuilt from the ground up, and in a way which respects the new and unique risks of our contemporary situation, and which protects and emphasises the responsibility of the individual citizen.
Key to this in our current predicament is to press on with critical questions pertaining to the obviously dangerous mass vaccinations and to investigate the corruption of our political and scientific institutions that the Covid situation has shed light on.
It is critical that we immediately begin to remedy the significant damage that has been rendered to global public health, and to the open society as such.
Johan Eddebo, Ph.D, researcher in digitalisation and human rights
Sture Blomberg, MD, Ph.D, Associate Professor in Anaesthesiology and Intensive Care and former senior physician
Ragnar Hultborn, Professor Emeritus, specialist in oncology
Sven Román, MD, Child and Adolescent Psychiatrist, since 2015 Consultant Psychiatrist working in Child and Adolescent Psychiatry throughout Sweden
Lilian Weiss, Associate Professor, specialist in surgery
Nils Littorin, resident in psychiatry, MD in clinical microbiology
The authors are members of the bio-medico-legal network of Läkaruppropet. They are organising a conference in Stockholm on January 21st-22nd in conjunction with the Swedish Doctors’ Appeal network. Its main focus will be on the consequences of the global COVID-19 politics and the effects of the Covid vaccines.
How COVID Vaccines Cause Cancer
Dec 30 2022
Antibodies are studied more than other immune proteins for association with disease. This does not mean that they are more decisive in disease outcomes. Type I interferon likely has far more impact.
Antibodies Are Not the Whole Story of Immune Resilience Toward Cancer
Much is being made of a recent study showing IgG4 antibodies spiking in the blood labs of those who are triple-injected with the mRNA COVID vaccines. Journalists are speculating that this may be the cause of increased cancers in the COVID-vaccinated. But that is not the main reason that the COVID-vaccinated are getting new cancer cases, sometimes aggressive “turbo cancers,” or coming out of remission from earlier cancer. Rather, there is earlier research that provides more plausible mechanisms for cancer risk, based on abundant prior knowledge of immune function. Let’s look at both the new study on IgG antibodies and earlier research.
The popular fallacy seems to be along these lines: ‘Antibodies are easy to test for. Plus, they are the focus of vaccine development and vaccine action. So therefore we spend a lot of time thinking and talking about them. So therefore they must be important markers of disease outcomes. So therefore they must be decisive in disease outcomes.’
After focusing my own work on cancer patients for the last 16 years as a naturopathic oncologist, if I made this mistake in thinking, most of my patients would be dead by now from misdirected efforts.
No, cancer remains a mega-problem of DNA damage, immune distraction, disrupted cell signaling, frenzied growth, lack of apoptosis, weakened tissues, angiogenesis, and metabolic derangement, as the principal features of an entity that feeds itself at the expense and to the detriment of the organ and the organism. These are the principal features of cancer, and they are hard as heck to treat successfully. I discuss that very daunting challenge here.
IgG3 Versus IgG4
First, let’s look at the new study on IgG3 versus IgG4 antibodies in the triple-jabbed. Herein, let’s call it the IgG4 study. It finds that the triple-jabbed may be developing a non-inflammatory tolerance to even high levels of spike proteins. That is, rather than having typical dyspnea, cough, olfactory and other full-blown COVID-type symptoms, IgG4 is a tolerant and tolerizing antibody that allows virions and spike protein load to accumulate in the body without the usual symptomatic alarms. Thus, a COVID+ PCR result with mild symptoms, or even no symptoms, often ensues. This may partly account for the many celebrities and politicians frequently quoted in MSM saying in so many words, ‘I tested positive for COVID, but thanks to my shots, it’s mild.’ Yet their lack of effective immune defeat of SARS-CoV-2 is what prevents their developing a lasting neutralizing immunity. So they (at least at first) tolerate high spike protein loads and are perpetually vulnerable to recurrent infections. Even more worrisome, what underlies that recurrence of mild symptoms, show the IgG4 study authors, is a precarious derangement of immune function with potentially problematic stockpiling of viral load, spike proteins and antibodies, with potentially devastating consequences for their future health outcomes. Even a myeloma like abundance of immunoglobulins can create a multiple myeloma-like disease in the COVID-vaccinated, a sludgy protein-laden blood that is harmful to the fine filtration structures, glomeruli, of the kidneys.
That immune deviation, misdirection and derangement has been previously described as pathogenic priming, a maladaptation of the immune system to either ignore or to fight ineffectively against genuine threats, while at the same time focusing its resources to slay the paper tigers of non-threatening antigens. This happened in the design of the mRNA vaccines to produce a spike protein that was characteristic of the original Wuhan strain of SARS-CoV-2, but turned out to be ineffective against Delta, Omicron and subsequent strains, as some of us had earlier warned. Because the Wuhan strain had already flamed and burned out, the COVID vaccines were obsolete by the time they were offered to the public.
Under circumstances of natural infection, whereas IgM antibodies flare for a short time after infection onset, IgG antibodies, in contrast, are slower to develop, and are those that remain long after an infection has resolved. (For example, my measles IgGs are still robust on a blood lab decades after I had measles as a child, with only natural immunity, no vaccine history.)
The subclass IgG4 is a non-inflammatory one that is correlated with tolerance to antigens, similar to allergy shots rendering the immune response more tolerant to grass pollen. IgG4 has no known effector function. Likewise, IgG4 seems to be inversely correlated with anaphylaxis. Here, in the IgG4 paper, regarding the COVID-vaccinated, IgG4 increases considerably, over 38 times, after a third mRNA injection. Please note that the scale of the y-axis is logarithmic, putting the IgG4s quite far up there.
At the same time, both triple- and double-jabbed lose a considerable amount of their IgG3 antibodies, discovered at 180-day and 210-day follow-up labs, respectively. Note again the logarithmic scale, showing cratering drop-off of IgG3 antibodies, with skyrocketing IgG4 antibodies. This is from Figure 1 of the IgG4 paper:
The subclass IgG3 is sometimes thought, including by the IgG4 authors, to be pro-inflammatory, one of many immune assaults against invading pathogens. Although there is evidence to the contrary as well. IgG3 is thus sometimes assumed, including by the IgG4 authors, and interested journalists, to neutralize, or fight effectively against, antigens.
However, there is little support, other than correlation of titers, for the assertion that IgG3 antibodies may be effective warriors against pathogens. The IgG4 study authors acknowledge an earlier observation of “IgG3 responses correlating with partial protection against HIV,” and only a rise in IgG3 antibodies after natural infection with SARS-CoV-2, such as reported here, without mechanism of its protection.
One possible clue as to the IgG4 study authors’ observations of low IgG3 is the glycosylation of IgG3 as impactful on SARS-CoV-2 infection severity. (Immunoglobulins are glycosylated protein molecules generally, but hyper-glycosylation seems to be a problem. Glycosylation is generally detrimental to its optimal function; notorious glycosylation has devastated more than simply antibodies, in our junk food loving culture.)
IgG3 antibodies are a very small proportion of IgG antibodies and have not yet been well-studied. Both IgG3 and IgG4 antibodies are generally a small proportion of all our B-cells, 3% and 4% respectively.
Low IgG3 antibodies are not necessarily correlated with low disease severity. For example, in COPD, we see this correlation of low IgG3 levels with life-threatening exacerbations of COPD. All antibodies, including IgG3 and IgG4, generally rise and then fall in case of natural infection. Below I will explain why I am not so sure the cause and effect vector goes as is currently being assumed, from low IgG3 / IgG4 ratio to generalized immune dysfunction. Rather, I suspect that it may more likely be an effect of other mechanisms, described below.
There Is so Much More to Immune Function Than Only Antibodies
The first problem with the current IgG fascination is the assumption that just because antibodies consume much attention, and are easily measurable proteins on a blood lab, that they are then necessarily impactful on the vast complexity of the rest of the immune system. Metaphorically, by assuming that that which we can see is necessarily decisive, we are looking at the skin, so to speak, and assuming that we therefore know the functions of the internal organs and that the skin is the dominant cause of internal effects. Obviously, such is not the case.
Let’s first assume that the highly mobile and ubiquitous blood contains many of the cells in our immune system and are, as a whole and in parts, key to optimal immune function. Here is the proportion of IgG immunoglobulin antibodies to the rest of the immune system:
Immunoglobulins are present on the surface of B-cells, where they act as receptors for antigens. B-cells fluctuate in number, but average 5.2% of all white blood cells. White blood cells are 0.1% of all cells in the blood. Therefore B-cells are about 0.00005% or 5 in 100,000 cells in the blood.
I explain further about that here.
This proportion of B-cells to other cells in the blood is vanishingly small. If you can see the very skinny red line at the far left of the band below, that is the proportion of all B-cells compared to the vast remainder of cells in the blood. (The thin red line would actually need to be a little thinner to be true to scale.)
Now let’s look at other aspects of immune function that are powerhouse fighters against cancer, but have been associated with high viral load and/or high spike protein, such as is expected to occur after COVID vaccination. These researchers found that two of our most important cancer-fighting cells, natural killer (NK) cells and CD8+ T-cells were significantly reduced in these circumstances. Reduction in NK cells is seen with more aggressive tumors.
But the major problem with the mRNA COVID vaccines and cancer risk was shown in April of this year, in the Seneff, Nigh paper.
The science community’s pre-occupation with the relatively smaller adaptive immune system, mostly its humoral portion, and unfamiliarity or disinterest in the vastly more important and stronger innate immune system has led attention away from this seminal paper. I have to recommend not only reading but thoroughly studying the Seneff, Nigh paper for the best understanding to date of the effect of the COVID vaccines on tumorigenesis, immune-failure with respect to cancer and metastatic events.
What Seneff et al found is that the most profound threat to immune function by the mRNA vaccines is the interference with Type I interferon signaling pathways. This in turn debilitates the surveillance capabilities of the immune system in cancer detection. As a result, we see both new tumors and metastases of existing cancers in the COVID-vaccinated. We see what is now called turbo cancers. Here is how Seneff et al supports that hypothesis. Their paper is enormously detailed, and my summary of it below is quite brief.
Ivanova, et al found that people who were naturally infected with SARS-CoV-2 have been able to dramatically up-regulate our arguably most crucial cytokine, Type I interferon, as seen from their peripheral dendritic cells, whereas mRNA-vaccinated people have not shown this ability, nor any such increase, nor any progenitor cells for the same. From those various findings, is evident that the COVID vaccines suppress Type I interferon signaling. The results are a devastating breakdown of many downstream immune functions, creating new vulnerability to not only viral diseases, but also to cancer. The necessity of interferons for the body’s war against cancer is further seen in the productive clinical use over decades of interferon as a therapeutic agent to cancer patients.
The most appreciated mechanisms of Type I interferon against cancers include up-regulation of the tumor suppressor gene p53, as well as kinase inhibitors, and the resulting arrest of cancer’s cell reproduction. Perhaps even more crucial is that Interferon-alpha, a type of interferon I, makes cancer recognizable, or in a way visible to other immune cells for destruction. Two other major effects of Type I interferons, specifically interferon-alpha, are cell differentiation and apoptosis, which are two of the major events that are important for a natural victory over cancer. Type I interferon also activates the essential cancer-fighting cells discussed above, CD8+ and NK cells. There are further genetic effects of Type I interferons, each of which tend to suppress tumors, notably through IRF-7 genes. These genes have impact on cancers of the breast, prostate, uterus, ovaries and pancreas. But these and oncogenes generally appear to become dysregulated by the mRNA vaccines.
Fay et al discuss G-quadruplex formations in RNA, and that role in proto-oncogene expression. This can in turn lead to cancer progression.
Cancer Incidence
Even before the boosters were rolled out to the public, the Vaccine Adverse Events Reporting System (VAERS) of the Dept of Health and Human Services (HHS) showed vastly more cancers following COVID vaccines than for all other vaccines during the 30-year history of VAERS. These new cancers following the COVID vaccines accounted for 98% of cancers reported. Here again from Seneff et al:
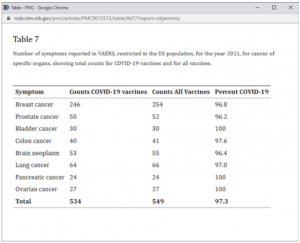
Seneff, Nigh et al https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9012513/
It should be noted that the reporting of these 2021 cancers occurred in large part prior to the US public’s (tepid) uptake of even the earliest mRNA COVID boosters (injection #3 in the fall of 2021) as here shown in Our World in Data.
That 3rd injection is the one after which the IgG4 paper authors saw the most difference in IgG3/IgG4 ratios, but not necessarily the greatest increase in cases of cancer.
Let’s consider the whole immune system, not only immunoglobulins, as necessary to protect against the ravages of cancer. Immune cells and cytokines, and their exquisitely coordinated and synergistic functions, must be protected from the destructive events initiated by irreversible experimental injections of novel products, such as the mRNA vaccines.
COVID Boosters Trigger Metastasis
Analysis by Dr. Joseph Mercola
January 05, 2023
STORY AT-A-GLANCE
- Cancer rates have increased since the introduction of the COVID shots and is now one of the top three leading causes of premature death among younger adults — a trend that in turn is driving down U.S. life expectancy
- The leading causes of death in 2021 were heart disease and cancer, both of which are potential side effects of the COVID jabs
- Dr. Angus Dalgleish, professor of oncology at St. George’s University of London, warns that COVID boosters may be causing aggressive metastatic cancers
- Research shows SARS-CoV-2 spike protein obliterates 90% of the DNA repair mechanism in lymphocytes, a type of white blood cell that helps your body fight infection and chronic disease, including cancer
- The COVID jab is less effective in lymphoma patients. Emory University researchers found only 68% of non-Hodgkin lymphoma and chronic lymphocytic leukemia developed neutralizing antibodies after the second dose, compared to 100% of healthy controls
Cancer rates have increased since the introduction of the COVID shots and is one of the top three leading causes of premature death among younger adults — a trend that in turn is driving down U.S. life expectancy.
In 2019, the average life span of Americans of all ethnicities was nearly 78.8 years.1 By the end of 2021, life expectancy had dropped to 76.42 — a loss of nearly three years, which is an astounding decline. The leading causes of death in 2021 were heart disease, cancer and COVID-19, all three of which were higher in 2021 than in 2020,3 and both heart disease and cancer are potential side effects of the COVID jabs.
COVID Boosters Are Triggering Metastatic Cancer
November 26, 2022, The Daily Sceptic published a letter4,5 to the editor of The BMJ, written by Dr. Angus Dalgleish, professor of oncology at St. George’s University of London, warning that COVID boosters may be causing aggressive metastatic cancers:
“COVID no longer needs a vaccine programme given the average age of death of COVID in the U.K. is 82 and from all other causes is 81 and falling,” Dalgleish writes.6 “The link with clots, myocarditis, heart attacks and strokes is now well accepted, as is the link with myelitis and neuropathy ...
However, there is now another reason to halt all vaccine programmes. As a practicing oncologist I am seeing people with stable disease rapidly progress after being forced to have a booster, usually so they can travel. Even within my own personal contacts I am seeing B cell-based disease after the boosters.
They describe being distinctly unwell a few days to weeks after the booster — one developing leukemia, two work colleagues Non-Hodgkin’s lymphoma, and an old friend who has felt like he has had Long COVID since receiving his booster and who, after getting severe bone pain, has been diagnosed as having multiple metastases from a rare B cell disorder.
I am experienced enough to know that these are not the coincidental anecdotes ... The reports of innate immune suppression after mRNA for several weeks would fit, as all these patients to date have melanoma or B cell based cancers, which are very susceptible to immune control — and that is before the reports of suppressor gene suppression by mRNA in laboratory experiments. This must be aired and debated immediately.”
New Norm: Explosive Cancer Relapses
In a December 19, 2022, article7 in Conservative Woman, Dalgleish continues discussing the phenomenon of rapidly spreading cancers in patients who were in stable remission for years before receiving their COVID boosters. He notes that after his letter to The BMJ was published, several oncologists have contacted him to say they’re seeing the same thing in their own practices.
“Seeing the recurrence of these cancers after all this time naturally makes me wonder if there is a common cause?” he writes.8 “I had previously noted that relapse in stable cancer is often associated with severe long-term stress, such as bankruptcy, divorce, etc.
However, I found that none of my patients had any such extra stress during this time, but they had all had booster vaccines and, indeed, a couple of them noted that they had a very bad reaction to the booster which they did not have to the first two injections.
I then noted that some of these patients were not having a normal pattern of relapse but rather an explosive relapse, with metastases occurring at the same time in several sites ... Scientifically, I was reading reports that the booster was leading to a big excess of antibodies at the expense of the T-cell response and that this T-cell suppression could last for three weeks, if not more.
To me, this could be causal as the immune system is being asked to make an excessive response through the humoral inflammatory part of the immune response against a virus (the alpha-delta variant) which is no longer in existence in the community.
This exertion leads to immune exhaustion, which is why these patients are reporting up to a 50% greater increase in Omicron, or other variations, than the non-vaccinated.”
A Change of Heart and Mind
Interestingly, in mid-2021, the Daily Mail published an article in which Dalgleish encouraged people to get the COVID shot, especially younger individuals.9 Dalgleish explains that, at the time, there was an “overwhelming push by the government and the medical community ... that this would be in everyone’s best interest.”
So, he caved to the narrative, even though he had concerns from the start. Now, however, the environment has changed and there’s really no need for these experimental shots anymore.
His concerns further grew when his son developed myocarditis “after having a jab he did not want but that he needed for work and travel purposes.” A friend of his son, who was in his early 30s, suffered a stroke after his jab, and a relative of a close colleague died from a heart attack at the age of 34 after hers.
“I began to be highly alarmed that it was the vaccines causing these symptoms,” Dalgleish writes,10 “and that just as we had written11 ... a genetically engineered virus had serious implications for vaccine design.
This paper, which was suppressed and therefore did not appear in print for many months, reported that the sequence of the virus was completely consistent with having been genetically engineered, with a furin cleavage site and six inserts at places that would make the virus very infectious, and the reason this had such tremendous implications for vaccine design was that 80% of these sequences had homology to human epitopes.
In particular, we had noticed a homology with platelet factor 4 and myelin. The former is also certainly associated with what is known as VITT (low platelets and clotting issues) and the latter associated with all the neurological problems, such as transverse myelitis, both of which are now recognized as side effects of the vaccine even by the MHRA [Medicines and Healthcare Products Regulatory Agency in the UK].”
Authorities Have Willfully Ignored All Warning Signals
Dalgleish says his team’s findings were eventually circulated among cabinet members and various medical committees, but everyone ignored them. As a result, many have been placed at unnecessary risk for serious injury and/or death.
As Dalgleish points out, young hearts over-express the ACE receptor that the virus was engineered to bind to. This binding with the ACE2 receptor is what “sets off the inflammatory response, which leads to myocarditis, pericarditis, stroke and deaths,” Dalgleish says.
This could explain the dramatic increase observed in deaths of young athletes who were jabbed: They simply have more ACE2 receptors that bind to the spike proteins created by the jab. Dalgleish continues:12
“When the facts change, or new facts emerge, the position of all those in authority directing mandates should change but unfortunately, they did not.
I tried desperately to point out that all the evidence that vaccines might have been useful in helping to curtail the pandemic was changing; that it was becoming very clear that there were highly significant side effects to the vaccine programme that Pfizer had gone to great lengths to cover up, and that it was only a court case in the US that led to them becoming available.
At this stage the whole vaccine programme should have been stopped but nobody seemed to want to address this, neither the Government, the medical authorities or the media.
Having written many articles for the Daily Mail arguing against lockdown and for it never to be used again, I was extremely keen to address my change of opinion on the vaccines and to warn people of their dangers particularly to younger people, and to point out there were no grounds at all for giving it to children.
Unfortunately, all my efforts and approaches to the mainstream media on this subject have been rejected. This, I believe, is something that will come back to haunt all those who introduced an Orwellian kind of suppression to the emerging truth, which labelled doctors trying to save their patients along the lines of ‘first do no harm’ as outcasts or villains.”
Scientific Proof COVID Jab Causes Cancer
Back in August 2022, The Exposé13 highlighted scientific evidence showing the COVID jabs can cause cancer of the ovaries, pancreas and breast, and that “a monumental cover-up is taking place to suppress the consequences ... on women’s health.”
Research shows SARS-CoV-2 spike protein obliterates 90% of the DNA repair mechanism in lymphocytes, a type of white blood cells that help your body fight infection and chronic disease, including cancer.
The research in question was that of Jiang and Mei, who published a peer-reviewed article showing the SARS-CoV-2 spike protein obliterated the DNA repair mechanism in lymphocytes, a type of white blood cells that play an important role in your immune system. Lymphocytes help your body fight infection and chronic disease, including cancer. Professional data analyst Joel Smalley writes:14
“The viral spike protein was so toxic to this pathway that it knocked 90% of it out. If the whole spike protein got into the nucleus (in the ovaries), and enough of it was produced and hung around long enough before the body was able to get rid of it all, it would cause cancer. Fortunately, in the case of natural infection, this is unlikely to occur.
Unfortunately, the experimental mRNA toxshot induces spike protein to be produced (the full-length spike exactly matching — amino acid for amino acid — the full length of the viral spike protein15) in and around the cell nucleus and is produced for at least 60 days and almost certainly longer.16
‘Fact checkers’ said the viral spike protein doesn’t get in the nucleus despite the expert scientists showing that it absolutely does. Public health authorities and regulators said the vaccinal spike protein doesn’t get in the nucleus despite the mRNA manufacturers submitting pictures of it doing so to them as part of their emergency use application ...
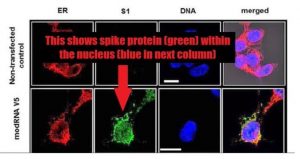
Jiang and Mei, quite logically and reasonably, cautioned that the mRNA spike protein would likely have the same effect as the viral spike protein on p53 and therefore cause cancer ... [The] Jiang and Mei paper was retracted due to spurious ‘expressions of concern’ (EOC) about the methods of the study despite them being standard practice ...
Well, despite the retraction, the spike protein circulating in large quantities, in the direct vicinity of the cell nucleus, for elongated periods of time, still has the potential to induce cancer in those cells (ovary, pancreas, breast, prostate, lymph nodes). These cancers can take years to develop and so it’s possible that we don’t see much of a safety signal for 5 or 10 years.”
As noted by Smalley, one of the authors of the EOC that led to the retraction of the paper was Eric Freed, Ph.D., who heads up the U.S. National Institutes of Health’s Center for Cancer Research.
He’s been a tenured investigator with the National Institute of Allergy and Infectious Diseases (NIAID) and NIH since 2002,17 the very agencies that funded Moderna’s mRNA jab, yet this conflict of interest was not disclosed in the EOC.
A Not so Rare Cancer Case
At the end of September 2022, The Atlantic18 featured the story of Belgian immunologist Michel Goldman, 67, who in the spring of 2021 got his first and second COVID shot. In the fall that year, he was diagnosed with lymphoma, cancer of the immune system.
Mere weeks after his body scan and diagnosis, he got his first booster, thinking he needed it since he’d soon become immunocompromised by the chemotherapy. But the booster caused a rapid decline in his health.
Another body scan at the end of September 2021, just three weeks after his first scan, revealed “a brand-new barrage of cancer lesions — so many spots that it looked like someone had set off fireworks inside Michel’s body,” Roxanne Khamsi writes:19
“More than that, the lesions were now prominent on both sides of the body, with new clusters blooming in Michel’s right armpit, and along the right side of his neck.
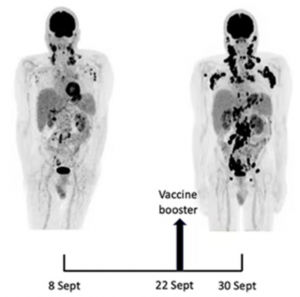
When Michel’s hematologist saw the scan, she told him to report directly to the nearest hospital pharmacy. He’d have to start on steroid pills right away, she told him. Such a swift progression for lymphoma in just three weeks was highly unusual, and he could not risk waiting a single day longer.
As he followed these instructions, Michel felt a gnawing worry that his COVID booster shot had somehow made him sicker. His brother [Serge, head of nuclear medicine at the hospital of the Université Libre de Bruxelles] was harboring a similar concern.
The asymmetrical cluster of cancerous nodes around Michel’s left armpit on the initial scan had already seemed ‘a bit disturbing,’ as his brother said; especially given that Michel’s first two doses of vaccine had been delivered on that side. Now he’d had a booster shot in the other arm, and the cancer’s asymmetry was flipped.
The brothers knew this might be just an eerie coincidence. But they couldn’t shake the feeling that Michel had experienced what would be a very rare yet life-threatening side effect of COVID vaccination.”20
T Cells Gone Berserk
Goldman, who was an early champion of the mRNA COVID shots, now “suspected that he was their unlucky victim,” Khamsi writes.21 He decided to go public about his cancer despite fears “anti-vaxxers” would use it to argue against the COVID jab. His concern for people who had the same type of cancer he had won out.
There are approximately 30 different subtypes of lymphoma. The kind Goldman had — angioimmunoblastic T-cell lymphoma — attacks follicular helper T cells, which play a crucial role in your body’s immune response to invading pathogens.
Helper T cells serve as a messenger between dendritic cells, which identify the pathogen, and B cells that make the appropriate antibodies. The mRNA COVID shots “are especially effective at generating that message, and spurring its passage through the helper T cells,” Khamsi writes.
This activation of helper T cells is part of what makes the COVID jabs work. But Goldman began to suspect that revving up those helper T cells might in some cases cause them to go berserk, resulting in tumors, or worsening of already existing ones.
Other Case Reports
Goldman was lucky. He lived to talk about it. Many others have not been so fortunate. And while he still believes he’s an “ultra-rare” case, he’s since received reports from other patients who suddenly developed angioimmunoblastic T-cell lymphoma after their shots. As reported by Khamsi:22
“Around the time of his February follow-up, Michel received a message from a doctor who had read his self-referential case report. The doctor’s mother had been diagnosed with the same subtype of lymphoma that Michel has following a COVID booster shot. More recently, he got an email from a woman whose sister had been vaccinated and received that diagnosis the following month.”
In August 2022, Frontiers in Medicine published a case report23 describing “rapid progression of marginal zone B-cell lymphoma” following the COVID jab. The 80-year-old Japanese woman featured in the report developed a noticeable tumor the very next day after her first shot. According to the authors:24
“Initially, we suspected head-and-neck benign lymphadenopathy as a side effect of vaccination. Nine weeks later, the number of swollen submandibular and parotid glands increased, and the lymph nodes further enlarged.
Finally, the right temporal mass was diagnosed as marginal zone B-cell lymphoma based on immunohistochemical and flow cytometry findings of biopsy specimens.
Our findings suggest that although 4-6 weeks of observation for lymph node inflammation after the second vaccination is recommended, malignancy should also be considered in the differential diagnosis of lymphadenopathy following vaccination.”
COVID Jab Is Far Less Effective in Lymphoma Patients
In May 2022, a single-center study25 at Emory University discovered that the humoral immune response in patients with non-Hodgkin lymphoma (NHL) or chronic lymphocytic leukemia (CLL) was significantly reduced after getting a COVID jab, compared to people who did not have either of those diagnoses.
Patients with NHL or CLL also didn’t have nearly the same antibody response to the shot. Only 68% of them developed neutralizing antibodies against SARS-CoV-2 after the second dose, compared to 100% of healthy controls. NHL/CLL patients who had undergone anti–CD20-directed therapies within one year of the first dose had the lowest antibody levels.
Turbo-Charged Cancers Are Becoming More Prevalent
Data from the Defense Medical Epidemiology Database (DMED)26 — historically one of the most well-kept and most heavily-relied upon medical databases in the world — showed that, compared to the previous five-year averages, cancer among Department of Defense (DOD) personnel in 2021 skyrocketed.
Overall, cancers tripled among servicemen and their family members after the rollout of the COVID shots. Breast cancer went up 487%. Exploding cancer rates are also seen elsewhere. One of the first to warn that the shots might cause cancer was Dr. Ryan Cole, a pathologist who runs his own pathology lab.
He suspects the shots accelerate already existing cancers by way of immune dysregulation.27 He noticed that cancers that were previously well-controlled would suddenly grow out of control and rapidly lead to death once they got the COVID jab.
Swedish pathologist, researcher and senior physician at Lund’s University, Dr. Ute Kruger, has also observed an explosion in rapidly advancing cancers in the wake of the COVID shots. For example, she’s noticed:28,29
- Cancer patients are getting younger — The largest increase is among 30- to 50-year-olds
- Tumor sizes are dramatically larger — Historically, 3-centimeter tumors were commonly found at the time of cancer diagnosis. Now, the tumors they’re finding are regularly 4 to 12 centimeters, which suggests they’re growing at a much faster rate than normal
- Multiple tumors in multiple organs are becoming more common
- Recurrence and metastasis are increasing — Kruger points out that many of the cancer patients she’s seeing have been in remission for years, only to suddenly be beset with uncontrollable cancer growth and metastasis shortly after their COVID jab
These “turbo-cancers,” as Kruger calls them, cannot be explained by delayed cancer screenings due to lockdowns and other COVID restrictions, as those days are long gone. Patients, despite having access to medical screenings as in years past, are showing up with grossly exacerbated tumor growths, and she believes this is because the cancers are being “turbo-charged” by the mRNA jabs.
Disturbingly, as detailed in “How Cancer Deaths From the COVID Jabs Are Being Hidden,” analysis of U.S. Morbidity and Mortality Weekly Report (MMWR) data suggests the U.S. Centers for Disease Control and Prevention has been filtering out and redesignating cancer deaths as COVID deaths since April 2021 to eliminate the cancer signal. The signal is being hidden by swapping the underlying cause of death with main cause of death.
Spike Protein Disrupting Immunity in Millions After COVID Infection or Vaccination: Here’s How It’s Being Treated
Pathology of Spike Proteins
Immune Cell Dysfunction
Spike Protein Causes Fatigue
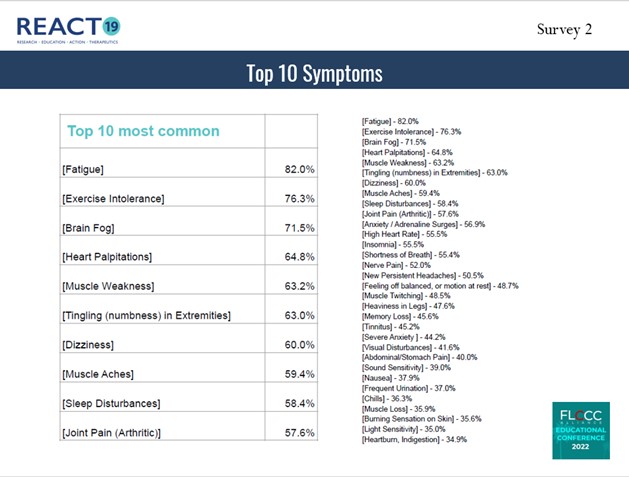
Spike Protein Damage to Blood Vessels and Organs
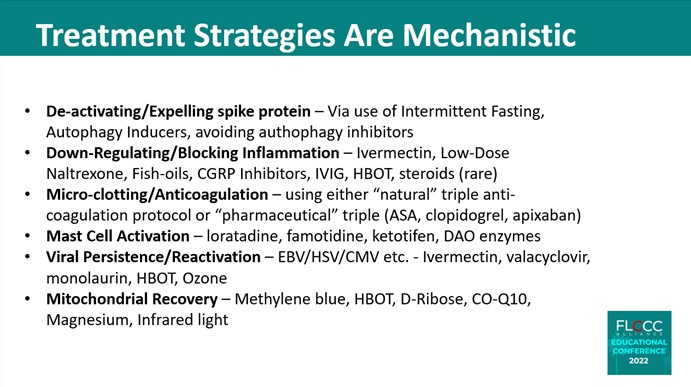
FLCCC’s First Line Treatments
Ivermectin
Low Dose Naltrexone
Resveratrol
Low Dose Aspirin
Melatonin
Differences Between Long COVID and Post-Vaccine Syndrome
VAERS COVID Vaccine
Adverse Event Reports
https://www.openvaers.com/covid-data
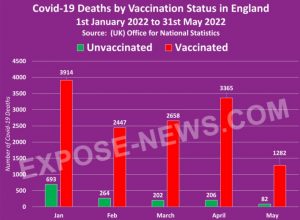
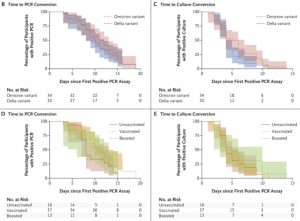 The study follows a host of similar reports and analyses conducted by researchers who deduced similar conclusions that undercut the efficacy of COVID-19 vaccines.Natural immunity has also proven to be superior in fighting against the initial infection of COVID-19.Despite these data points, which have been routinely censored by social media “fact-checkers,” the White House and mainstream media have continued to uncritically promote COVID-19 vaccines.The embrace of COVID-19 vaccines by companies including Pfizer and Moderna follows massive lobbying campaigns and spending efforts by the pharmaceutical giants.
The study follows a host of similar reports and analyses conducted by researchers who deduced similar conclusions that undercut the efficacy of COVID-19 vaccines.Natural immunity has also proven to be superior in fighting against the initial infection of COVID-19.Despite these data points, which have been routinely censored by social media “fact-checkers,” the White House and mainstream media have continued to uncritically promote COVID-19 vaccines.The embrace of COVID-19 vaccines by companies including Pfizer and Moderna follows massive lobbying campaigns and spending efforts by the pharmaceutical giants.
The "safe and effective" narrative is falling apart
Here is my list of over 25 leading indicators that the momentum is moving in our favor. I'd be surprised if the narrative doesn't fall apart soon. It's now unravelling quickly in the UK.
60,000 scientists call for an end to mass vaccination

The scientific community speaks out against the health policy of the authorities: the declaration of Great Barrington (USA) against massive injections of mRNA has collected 870.000 signatures.
While the President of the French Republic meets this Monday, December 6, a new Council of Defense to fight against the spread of the epidemic of Covid-19, more and more voices are raised against the health policy completely crazy of the authorities which aims to vaccinate more and more the population. After the third dose of vaccine imposed, under penalty of deactivation of the health pass! There is now the question of vaccinating children from 5 to 11 years old. A decision that would be very serious according to many doctors and researchers that we have reported here.
Serious side effects
Mass vaccination with a still experimental messenger RNA vaccine has long worried the scientific community. As of October 4, 2020, three high-level scientists, Prof. Martin Kulldorff, professor of medicine at Harvard University, biostatistician and epidemiologist, specializing in the detection and monitoring of infectious disease outbreaks and in the evaluation of vaccine safety. Dr. Sunetra Gupta, professor at Oxford University, an epidemiologist specializing in immunology, vaccine development and mathematical modeling of infectious diseases. And Dr. Jay Bhattacharya, a professor at Stanford University School of Medicine, a physician, epidemiologist, health economist, and public health policy expert specializing in infectious diseases and vulnerable populations, recommended an alternative approach to Covid-19 in what was called the Great Barrington Declaration. Because they are very concerned about the side effects of mass vaccination on populations.
What do they advocate? A “focused” protection. That is to say, to protect as much as possible the elderly, the infirm, the frail and to leave the others to live normally until the society reaches the collective immunity. However, mass vaccination by mRNA makes this collective immunity impossible.
One of the original co-signatories was Dr. Simon Thornley, an epidemiologist and biostatistician at the University of Auckland. The Great Barrington Declaration has since been signed by 60,000 physicians and scientists and is becoming increasingly well known around the world. A petition has so far gathered 870,000 signatures of support.
The Great Barrington Declaration
“The Great Barrington Declaration – As infectious disease epidemiologists and public health scientists we have grave concerns about the damaging physical and mental health impacts of the prevailing COVID-19 policies, and recommend an approach we call Focused Protection.
Coming from both the left and right, and around the world, we have devoted our careers to protecting people. Current lockdown policies are producing devastating effects on short and long-term public health. The results (to name a few) include lower childhood vaccination rates, worsening cardiovascular disease outcomes, fewer cancer screenings and deteriorating mental health – leading to greater excess mortality in years to come, with the working class and younger members of society carrying the heaviest burden. Keeping students out of school is a grave injustice.
Keeping these measures in place until a vaccine is available will cause irreparable damage, with the underprivileged disproportionately harmed.
Fortunately, our understanding of the virus is growing. We know that vulnerability to death from COVID-19 is more than a thousand-fold higher in the old and infirm than the young. Indeed, for children, COVID-19 is less dangerous than many other harms, including influenza.
As immunity builds in the population, the risk of infection to all – including the vulnerable – falls. We know that all populations will eventually reach herd immunity – i.e. the point at which the rate of new infections is stable – and that this can be assisted by (but is not dependent upon) a vaccine. Our goal should therefore be to minimize mortality and social harm until we reach herd immunity.
The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk. We call this Focused Protection.
Adopting measures to protect the vulnerable should be the central aim of public health responses to COVID-19. By way of example, nursing homes should use staff with acquired immunity and perform frequent testing of other staff and all visitors. Staff rotation should be minimized. Retired people living at home should have groceries and other essentials delivered to their home. When possible, they should meet family members outside rather than inside. A comprehensive and detailed list of measures, including approaches to multi-generational households, can be implemented, and is well within the scope and capability of public health professionals.
Those who are not vulnerable should immediately be allowed to resume life as normal. Simple hygiene measures, such as hand washing and staying home when sick should be practiced by everyone to reduce the herd immunity threshold. Schools and universities should be open for in-person teaching. Extracurricular activities, such as sports, should be resumed. Young low-risk adults should work normally, rather than from home. Restaurants and other businesses should open. Arts, music, sport and other cultural activities should resume. People who are more at risk may participate if they wish, while society as a whole enjoys the protection conferred upon the vulnerable by those who have built up herd immunity.”
Hard Data Shows the Covid Vaccines Don’t Work
The last several months have seen a heated debate about the effectiveness of the vaccines that are being currently administered against Covid-19.
The question on many people’s minds is: Do these pharmaceuticals work?
Both sides tend to feel quite strongly about their position which gives rise to a great deal of emotion as the debate goes on.
The good news is that being nearly a year into the vast vaccination enterprise we now possess sufficient data to determine whether the shots are effective or not.
As we know, the objective of vaccination is to eliminate or significantly reduce the incidence of the targeted disease. If a vaccine works, then in a highly vaccinated population we will see either complete elimination of the disease or a significant decrease of its incidence.
Since it is usually not practicable to achieve 100 percent inoculation rate, the question is what is the vaccination level that will either bring the disease under control or eliminate it altogether?
This level is sometimes referred to as “herd immunity.” We were told at first by experts, most notably Dr. Anthony Fauci, that the vaccination rate of 60 to 70 percent would confer herd immunity in regard to Covid 19.
Fauci’s position was roughly in line with our experience with many other diseases where such levels of inoculation have either eliminated them or made them endemic, i.e., sufficiently limited so that they do not pose a large-scale, epidemic-level threat to the community.
Some twelve months into the worldwide vaccination drive there are now a number of countries with vaccination rates of between 60 and 70 percent. There are also some countries and geographical areas with rates of 80 percent or above.
While we do not know the precise figure which would confer herd immunity against this disease, we can be sure of one thing: if the vaccines are effective, vaccination rates of more than sixty percent should result in a significant reduction in its incidence.
[NOTE: The effect of the vaccines is further augmented by natural immunity which, according to some experts, may run as high as 50% in some populations. Nearly two years into the pandemic, populations in many places have been extensively exposed to the virus and as a result possess natural antibodies. Therefore, inoculating, let’s say, 65 percent of the population with a good vaccine should result in overall immunity in excess of 80 percent. With this kind of immunity level we should expect, if not elimination of the disease, then certainly a considerable decline in its occurrence.]This, however, is not at all what has happened in most of the highly vaccinated countries and regions. What has transpired in many of them was the very opposite. Following the “success” of their vaccination drives, there occurred dramatic surges of Covid 19. Even more astonishingly, several of these countries posted a record number of cases just after achieving their very high vaccination figures.
This news may come as a shock to many people because the connection between high vaccination rates and subsequent explosion of cases has been virtually ignored by the mainstream media.
We will show you the reality of the situation by presenting the relevant data in an easy-to-see, straightforward way. We do this by juxtaposing graphs that depict vaccination rates with graphs that show case rates in countries with high vaccine uptakes.
Neither side in the debate would dispute the validity of the data presented below. The data is taken from the Google Coronavirus Statistics tool, which draws its material from official sources and government databases. The data is publicly available and widely accessible. If you wish to verify or reproduce the data used in this piece, you can do so easily by going to google.com and typing “coronavirus” plus the name of the country whose statistics you want to examine. Once the country’s data comes up, you can choose in the horizontal menu that runs right under the term “Statistics” what graph you want to see: “New Cases” or “Vaccinations.”
Gibraltar
On November 17, Gibraltar posted its highest number of new cases in more than 10 months. The surge became a cause of great concern and prompted the government to call off Christmas festivities. The last time Gibraltar had so many cases was at the height of the winter wave in mid-January of 2021.
(Gibraltar data via Google.com link)
The most startling aspect of the current surge is that Gibraltar is the most highly vaccinated region in the world with more than 99 percent of its population being fully vaccinated. Even more astonishingly, more than 40 percent of Gibraltarians have already received their booster.
Given what we have been told about the vaccines by the corporate media and government officials, you would be justified in thinking that this is some kind of misinformation or error. It is, however, an undeniable fact that here, in the very midst of Gibraltar’s current surge, 99 percent of its residents are fully vaxxed. This is something you can see for yourself in the chart below.
The example of Gibraltar should stand as a clear lesson and a dire warning to health officials and politicians around the world who are trying to force their populations into high vaccination uptakes. Gibraltar clearly shows that even a 99 percent vaccine rate followed by intense boostering will not tame or eliminate Covid 19 from the population. Quite to the contrary, it can coincide with near-record spikes that will likely surpass previous highs, especially as in the weeks ahead the country enters the winter period.
Singapore
By October 26 nearly 83 percent of the Singapore population received their course of Covid injections. This means that more than 8 out of 10 Singaporeans had achieved fully vaccinated status.
(Singapore data via Google.com link)
Singapore’s very high vaccination rate, however, did nothing to decrease the presence of the disease in the nation. The exact opposite, in fact, happened. On October 27, 2021, Singapore posted its record case count of 5,324 new cases.
This figure was nearly 300 percent higher than the previous record of 1,426 that occurred on April 20, 2021. At that time Singapore’s vaccination rate was only 15 percent.
If the vaccine were even remotely effective such a situation could have never arisen. There is simply no way that a country where 83 percent of population received an effective vaccine could ever experience such a record-breaking surge. Rather than an explosion of Covid, Singapore’s very high vaccination rate should have brought about herd immunity.
Denmark
As of November 12 Denmark’s vaccination stood at more than 75 percent.
(Denmark data via Google.com link)
On the same day, Denmark recorded 4,585 new cases of Covid 19, which was the country’s new case record. Denmark’s previous record was 4,508 cases posted in December of 2020 at the height of last year’s winter wave.
At the time of the old case record, the vaccination rate in Denmark was 0 percent.
The country’s very high vaccination rate not only did not eliminate the disease, but it coincided with record-breaking case numbers. If the vaccines injected into the bodies of two-thirds of Danes were any effective such a situation could have never come about.
Ireland
On November 16 of this year, Ireland boasted a vaccination rate of more than 75 percent.
(Ireland data via Google.com link)
On that day, Ireland posted 8,965 new cases of Covid-19. This was a new high for the nation.
The previous high was recorded on January 8, 2021. The figure stood at 8,227 then. At the time, Ireland’s vaccination rate was 0 percent.
Vaccinating 4.5 million people in Ireland – more than two-thirds of the population – was accompanied by an explosion of cases and a new case high for the nation.
Iceland
On November 16, 2021, the country’s vaccination rate stood at 76.4 percent.
(Iceland data via Google.com link)
On November 15, 2021, Iceland posted 420 new cases of Covid-19. This was a record which topped the country’s previous high by 500 percent when the country’s vaccination rate was zero.
Cayman Islands
On November 12, 2021, the country’s rate of fully vaccinated stood at 83.9 percent of the total population.
(Cayman Islands data via Google.com link)
On the same day, Cayman Islands posted 953 new cases of Covid-19, which was a new high for the country.
It exceeded the country’s high of 258 cases by more than 300% from the same day in the previous year. At that time the vaccination rate was zero percent.
Germany
On November 17, 2021, the country’s vaccination rate was 67.7 percent of the total population.
(Germany data via Google.com link)
On that day Germany posted 68,366 new cases of Covid-19. This set a new record. It exceeded by nearly 50 percent the previous high of 45,333 cases from January 2021. At the time of the previous high, the vaccination rate in Germany was 0 percent.
Austria
On November 19, 2021, country’s full vaccination rate stood at 65 percent.
(Austria data via Google.com link)
On the same day, Austria posted 15,809 new cases of Covid-19. This was a new high. It surpassed by more than 50% the country’s previous record of 9,586 cases from November 13, 2020. At that time, the vaccination rate in Austria was zero percent.
Vermont
On November 10, 2021, the state’s vaccination rate stood at 71%.
(Vermont data via Google.com link)
At the same time, the state of Vermont posted 611 new cases of Covid-19, which was a new high. It topped by more than 100% Vermont’s previous record of 277 cases reported on January 2, 2021. At that time, the vaccination rate in Vermont was less than 1 percent.
Israel
On September 13, 2021, country’s full vaccination rate stood at above 60 percent.
(Israel data via Google.com link)
On the same day, Israel posted 11,800 new cases of Covid-19. This was a new case high for the country in this pandemic. At the time Israel was a global leader in vaccine administration and held out as an example for the rest of the world. Yet at the same time Israel’s infection rate was the highest on the planet. The situation became so dire that Israel’s rate of infection was more than 50 percent higher than that of the second ranked country in that metric, Mongolia. As to the question of vaccine effectiveness, it was quite revealing that Israel led the world in vaccination as well as infection.
Israel’s September record exceeded by nearly 50% the country’s previous high of 7,305 cases posted on January 28, 2021. At the time of the previous high, the vaccination rate in Israel was 18.3%.
CONCLUSION
We have seen again and again record case counts in countries and regions with high vaccination rates. This shows that high vaccine uptake does not reduce the incidence of Covid 19.
Not only do the vaccines not lower the incidence of this disease, they tend to correlate with its increase. As we have seen above, a number of countries have experienced record-breaking surges right after achieving high inoculation rates.
Countries with vaccination rates of 65 percent or more should definitely not be in the pandemic or suffer surges. Yet they do, because “breakthrough” infections in the vaccinated are now very common and frequent. We do know for a fact that the vaccines do not prevent people from getting infected. This was confirmed in August by CDC Director Rochelle Walensky who openly admitted in a CNN interview that the vaccines can longer “prevent transmission.”
With winter coming there is every reason to be deeply concerned, since the high vaccination rates and accompanying explosion in cases were for the most part achieved in the summer and early fall when the virus is weak. As countries in the northern hemisphere are entering the winter season and the death counts keep going up quickly, we seem to stand on the brink of an extremely difficult period in the months ahead.
This situation exists despite the fact that many countries have achieved vaccination rates close to 70 percent. Europe’s average rate of full vaccination, for example, currently stands at 65.5 percent while 69.9 percent of Europeans have received at least one injection.
Europe’s high vaccine uptake falls well within the herd immunity range specified earlier this year by Dr. Fauci and other experts. With such an inoculation rate the pandemic should be if not over, then definitely under control. Instead, it is out of control.
Many European nations, as well as countries in other parts of the globe, are sounding the alarm and imposing a new wave of lockdowns.
If the vaccines were even remotely effective, this could have never happened in highly vaxxed territories.
The vaccines have not only failed to live up to their promise, but the data indicates that in a number of places they have made the situation worse by bringing about surges.
The data clearly demonstrates that the vaccines do not have the effect they were supposed to have. The figures and graphs above provide hard evidence of vaccine failure.
For governments to pursue high vaccination rates with the obviously ineffective vaccines is misguided and counterproductive. It is also highly irresponsible and dangerous because of the vaccines’ extensive and severe side effects.
The COVID Vaccine Failure
The CDC narrative on COVID is simply put: (1) the mask is your best defense, (2) COVID is a pandemic of the unvaccinated, and (3) vaccines are our only exit strategy. However, whenever their narrative falls apart, the emphasis shifts, cover-up lies become bolder, and dissenting science is censored.
According to CDC Director Rachel Walensky in a Twitter public service announcement, masks can help prevent the spread of COVID or flu or common cold by reducing your chance of infection by 80%. Yet eleven Randomized Controlled Trials (RCT), including a Danish RCT studying COVID, failed to show that masks prevent transmission of respiratory viruses. In two lawsuits the Ontario Nurses Association (ONA) won litigation against hospitals’ “vaccinate or mask” policy when RCT showed “scant evidence” that wearing masks reduced influenza transmission. Empirical evidence, including CDC’s own study, Oster's U.S. schools data, and comparing states with and without mask mandates failed to show a benefit to masking on COVID case rates. Though not reported in the media, the “mask is your best defense” isn’t settled science -- the WHO states that: “the use of masks by healthy people in the community setting is not yet supported by high quality scientific evidence.” Despite exclusive pro-mask news, parents are protesting against governors and school boards mandating masking K-12 students.
After vaccine uptake slowed, Walensky called COVID a “Pandemic of the Unvaccinated” to shame the unvaccinated into getting the vaccine. News reports touted divisive messaging of Biden-voting blue states as having higher vaccination rates and lower positivity rates than red states. However, her lies were contradicted when territories that were 80%+ vaccinated had the highest case rates -- Singapore with 85% vax rates, Iceland 92% vax rate, and Gibraltar with 100% vax rate saw peaking numbers. Compared to Florida case rates, California is 200% higher, NYC is 300% higher, the most vaxxed state Vermont is 700% higher. Of note: the most vaccine-hesitant were PhDs.
To explain the rising cases in highly vaccinated areas, Walensky reported that breakthrough infections were rare, representing < 1% of hospitalized infections, an assertion that contradicts CDC’s unpublished report showing 15% of May 2021 COVID cases were fully vaccinated, a 500% increase from April 2021, with decreased vaccine efficacy (VE) in nursing-home residents. Their <1% number was criticized for tallying all hospital cases from January to April 2021, well before most residents were vaccinated. Beginning May 1, CDC suppressed vaccinated positive case data -- they no longer published vaccine breakthrough cases, recommended against testing asymptomatic vaccinated individuals, and defined unvaccinated as someone within 14 days of receiving the COVID jab. The CDC’s definition of vaccine changed from “produce immunity” to “produce protection” against disease; their narrative became “vaccines can’t prevent transmission, but do prevent severe illness and death.”
When coercion and shaming didn’t increase vaccination rates, the Biden administration instituted a series of workplace vaccine mandates to compel COVID vaccines for employees in health care, government, and private corporations. The vaccine mandates contradict science by not accepting natural immunity (as EU does), by exempting politically connected groups (Congress and USPS), and administering a “leaky” vaccine. The mandated vaccine caused police and healthcare workers (HCW), many with natural immunity, to stand up for freedom and risk losing jobs. Their sacrifice was noticed when vaccine mandates caused hospital closures from nurses being fired and urban violence from firing policemen.
As COVID cases continued to rise, the CDC’s narrative shifted from vaccines being “safe and effective” to “get a booster shot for protection.” This follows Anthony Fauci announcing “waning of immunity not only against infection but against hospitalization and to some extent death, which is starting to now involve all age groups. It isn’t just the elderly.” An Israeli paper on booster shot protection shows that VE dropped to 71% in September and 60% in October. Two senior FDA vaccine officials resigned after Biden, FDA, and CDC heads announced a booster eligibility timeline prior to FDA approval. They explain their resignation in a Lancet paper -- existing data doesn’t warrant boosters, third dose decisions warrant more analysis of benefit-harm-population efficacy, and booster decisions “should be made by reliable science rather than by politics.” Like HCWs, police, and firefighters, they protested against having their dissenting views sidelined and censorship of physician scientists.
Since vaccines are the only exit strategy, news was suppressed on potential early treatment strategies, COVID’s low risk to children, alternate strategies to lockdowns, and vaccine side effects. The NIH could have led by initiating RCTs on early treatment regiments, face masks, natural immunity, and vaccine-induced myocarditis. Instead, physicians now lose their medical licenses for prescribing FDA approved drugs, like ivermectin, for off-label uses that 200+ members of Congress, Japan, and India used successfully.
Disregarding natural immunity was the most blatant, dangerous CDC lie, since many who refuse vaccination understand that prior infection is more protective. Furthermore, by not separating prior infected from the vaccinated and unvaccinated cohorts, the VE becomes meaningless. Only the most blind refuse to see that first responders, who put their lives on the line in the early pandemic to treat COVID patients and quell riots, are now sacrificing their livelihoods to refuse vaccines they don't want -- many were infected early while working tireless hours to protect Americans and now have lost jobs in the midst of hospital and police worker shortages.
As Americans, we can either be subject to medical political tyranny or stand for freedoms derived by our God-sanctioned morality. We need to decide whether to fight for our country founded as “a city on a hill” by the courageous who love freedom. Our alternative is a biosecurity state that promises us safety, tells only noble truths, and censors all dissenters. Now is the time to decide: a house divided cannot stand.
UK Data Shows No All-Cause Mortality Benefit for COVID-19 Vaccines
The Vaccine Wars Part I
| Mathew Crawford
|
The original title of this article was, "Did Alex Berenson Fall for a Simpson's Paradox?" I'm rewriting the title and introduction to this article after having finished an analysis that demonstrates no all-cause mortality benefit to the vaccinated in the UK. You'll have to read through the story to understand why.
Are the vaccinated dying at two-to-three times the rate of the unvaccinated in the UK as Alex Berenson's recent article suggests?
Let's be clear: Alex Berenson has done an excellent and courageous job during the pandemic. I follow his work and recommend that you do as well. He definitely digs into data better than most journalists, though we should not expect for him to do everything and be everything. He doesn't have to be perfect and you don't have to agree with every take to understand how important his work is. I am writing this before I analyze the data. However, I will note that my first instinct is to think that the [twice] Vaccinated cohort has an older age profile than the Unvaccinated cohort. Thus, we should expect to see greater all cause mortality in that group. This means what we are seeing is an illusion of data aggregation often called a Simpson's paradox, something I've written about several times before (here and here).
If you don't want to read through my discussion of this particular statistical illusion, skip down to the next section to read the conclusion.
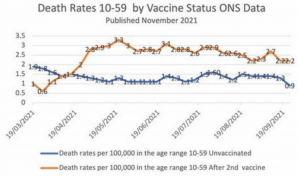
Let's be clear: I've seen statisticians and Wall Street quants at the highest levels fall for a Simpson's paradox here or there. Recognizing the way an aggregate can or should be disentangled is a different job than just doing the statistics. It often takes domain knowledge (and humility in reining in expectations) in addition to statistical awareness to spot a Simpson's paradox. Reversed trends in aggregates have probably fooled every human that walked the planet at one point or another, assuming they were numerate enough to view any data at all.
When I teach my Combinatorics, Probability, and Statistics courses, I stop and spend 10% of every course at every level on Simpson's paradox's and similar conditional data twists alone, giving students a chance to noodle over the strange "contradictions" in the results. This is from curriculum I've used with students ages 10-70:
This story is based on circumstances similar to those I've personally witnessed in baseball statistics. Here is the punch line: Peakoles is clearly the superior hitter (at least so far as batting average is concerned):
I spend so much time in class focusing on this kind of paradox and conditionality in probability and statistics for several reasons:
- I want for students to understand that problems aren't always straight-forward.
- Understanding these problems is the difference between an elite professional data geek and somebody with enough knowledge to be dangerous (which appears to be the substantial majority of the medical profession and all but the most elite epidemiologists during this pandemic).
We may reach a point at which having more problem solvers trained in teasing apart this kind of data results in millions of lives saved and hundreds of billions or even trillions of dollars saved annually in recovered lost productivity associated with poor management and governance decisions.
Modeling the UK Data Shows No All-Cause Mortality Benefit
I wrote the first half of this article prior to modeling the data.
I took the period mortality tables from the UK's Office of National Statistics (ONS). Next, I painstakingly (yuck) estimated every relevant point on the cumulative vaccinated chart by age from the UK's vaccination surveillance report, using some reasonable interpolations along the way. Then I realized that I needed population proportions for the subgroups of the age 10-59 cohort (Can we get a little granularity, please? Or just open source data?). Now, I can put together a projected mortality profile for each group during an ordinary year using weighted averages (where each age group's projected mortality gets multiplied by its proportion within each cohort, and the results are summed) for each week.
I then took the raw data Berenson pointed to out to one more decimal place, and plotted the actual 2021 all cause mortality data versus the expected all cause mortality data. As we can see, the Vaccinated cohort, due to having a generally older age profile, was expected to have higher all cause mortality. (Lighter hues are projected, heavier hues are what actually happened.)
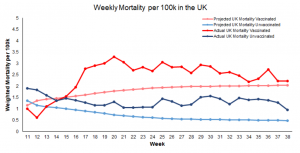
So, Alex was wrong to suggest that the data showed prima facie higher mortality in the Vaccinated cohort due to the vaccines. However, this result is quite interesting! It's hard to look at these graphs and easily determine which cohort has suffered more excess mortality during the middle months of 2021! So, I took the excess mortality from each cohort for each week, and also cumulatively, and plotted them:
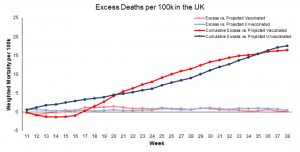
The cumulative trends go back-and-forth, and it seems reasonable to dismiss any difference as statistical noise. But when we do compute the tiny overall observed benefit at the end of the 28 week stretch to the vaccinated group, it amounts to a mere 5 deaths per million doses (at over $6 million per life saved).
I wonder who could have predicted all this?
Also, understand that the single dose all cause mortality rates in the 10-59 range were higher than for either of these cohorts, which is to say that the Unvaccinated cohort is doing just a tiny bit better than the average UK citizen during this time span! This is all very consistent with my hypothesis that the vaccines have no real efficacy. Even worse, they have come at the expense of hundreds of thousands of serious adverse events in the UK alone (possibly over a million at this point), and several billion pounds from the public treasury.
It's also unclear exactly how the laundering of post-vaccination mortality plays into official mortality categorizations, but the charts above are made playing by their rules, with their data. All this provides us with a frightening, if illuminating look at what the Kunlangeta are doing during (provoking?) an international governance and economic crisis.
Addendum: Note that I still think there are excess deaths among the vaccinated group not visible in this time frame because they occurred en masse at the outset of the UK's vaccination program among the elderly. It certainly appears that these vaccines are killing more people than they save.
Addendum 2: In response to a question on Twitter…from March 13 and for several months, the oldest among the 10-59 age group get vaccinated faster (think in proportions), while the youngest get vaccinated slower, which is why the age profile of the Vaccinated cohort grows (and the Unvaccinated gets lower). Hopefully this visualization helps.

Addendum 3: Note that the 10-16 year olds remain almost entirely unvaccinated through week 38. As the older groups shift into the Vaccinated cohort, this distills a lower mortality rate among the Unvaccinated cohort.
NULLIFYING NUREMBERG
Published August 4, 2021 | By Pennel Bird
Nazi War Criminals at Nuremberg Trials
[The HFR of July 23 explained how Xiden and leaders of other countries are committing Crimes Against Humanity with their vax mandates:
“Due to the horrific experimentation on Auschwitz prisoners by Nazi doctors such as Josef Mengele… it is has long been considered by international legal consensus a ‘Crime Against Humanity’ to force people against their will to take an experimental drug – which all Covid vaccines are.”
Here, TTP reader Pennel Bird provides a valuable further explication of why so many government leaders and officials are criminals violating international law.]
Dr. Josef Mengele is one of the true monsters of history. His profoundly evil medical experiments on Jews, the disabled, the mentally impaired, and other Third Reich deplorables are the stuff of pitch-black fever dreams.
Combining disparate elements of pernicious ideologies including eugenics, antisemitism, racial purity, and the German ideal of lebensraum (“room for living”), what eventually became Nazism informed Hitler’s ethos as he rose to power. His malignant weltanschauung eventually coalesced into the Final Solution.
Hitler’s infernal vision metastasized quickly to infect the belief systems of top-tier Nazis. Among other atrocities, Mengele used injections to attempt to change the eye color of his “patients” to blue to render them more Aryan. When these experiments went sideways, the fiendish M.D. demonstrated a penchant for “tidying up.” One person testified to having witnessed the diabolical doctor kill fourteen sets of twins in one night with chloroform injections to the heart in order to make comparative post-mortem observations.
After the Allies won the war, the Nuremberg Trials were convened to assess the astonishing breadth of the human tragedy as authored by Hitler and his henchmen and mete out punishment for their actions.
The cruelty and depravity of Mengele and others, including Adolf Eichmann, shocked the world, were almost beyond reckoning, and subsequently inspired the establishment of the Nuremberg Code — which was tacitly endorsed by nearly every nation on earth.
The ten points of the Nuremberg Code for human experimentation are as follows:
- The voluntary consent of the human subject is essential. This means that the person involved should have the legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over-reaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved, as to enable him to make an understanding and enlightened decision. This latter element requires that, before the acceptance of an affirmative decision by the experimental subject, there should be made known to him the effects upon his health or person, which may come from his participation in the experiment.
- The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study, and not random and unnecessary in nature.
- The experiment should be so designed and based on the results of animal experimentation.
- The experiment should be so conducted as to avoid all unnecessary physical and mental suffering and injury.
- No experiment should be conducted where there is an a priori reason to believe that death or disabling injury will occur.
- The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.
- Proper preparations should be made, and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability, or death.
- The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.
- During the course of the experiment, the human subject should be at liberty to bring the experiment to an end if he has reached the physical or mental state where continuation of the experiment seems to him to be impossible.
- During the course of the experiment, the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of the good faith, superior skill, and careful judgment required of him that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.
All COVID vaccines emergency authorized by the FDA have not yet been approved and are experimental, as they have undergone no long-term safety trials. Safety data are being partially collected in real time as adverse events are reported to VAERS. The tenets of the Nuremberg Code apply here as these vaccines are — by definition — a medical experiment being administered worldwide.
Given that, the explicit statement in #1 that subjects not be subject to coercion raises the question: does the threat of losing one’s job, not being able to attend college, or not being able to travel or attend a live event constitute coercion?
Does the president of the United States urging Americans to “get the shot” and then threatening to send emissaries door to door to “encourage” it constitute coercion?
Is it coercion to establish two Americas in which one half that chooses vaccination gets to eschew masks and move about freely, while the other half that doesn’t must stay masked and have their essential freedoms proscribed?
Later in #1, the text explicitly states that the subject must be made aware of “the effects upon his health or person.”
This is known as informed consent: patients need to be made fully aware of the potential dangers of a medical procedure in keeping with the Hippocratic oath.
There have been many reports of adverse events from the COVID vaccines, including Bell’s palsy, seizures, blood clotting, heart inflammation, and death. How many people reading this who got the vaccine had these potential side effects explained to them before getting jabbed? How many were afforded informed consent?
The second bullet point dictates the experiment (COVID vaccine) “should be so designed and based on the results of animal experimentation.” In an alarming break with decades of convention, the Pfizer and Moderna animal trials were run concurrently with human trials.
The human trials were not a result of animal trials, giving the manufacturers a chance to make safety adjustments, which constitutes a violation of the Nuremberg Code.
The fifth bullet point states that no experiment (the experimental vaccine in this case) should be given if there’s reason to believe it could cause a disabling injury or death.
With over 400,000 adverse events and 9,000 unconfirmed deaths from COVID vaccines reported to VAERS, is there reason to believe the COVID vaccines violate the Nuremberg Code in yet another way?
The eighth bullet point emphasizes the “highest degree of skill and care” by “scientifically qualified persons” when administering the vaccine. Do pharmacists fit that profile? Do school nurses? How about the folks jabbing people motoring through drive-thru clinics?
Does the fact that they all enjoy total liability protection from vaccine injury and death give pause?
The last bullet point emphasizes that the administering agent should exercise caution in fulfillment of the Hippocratic oath by terminating treatment if there is reason to believe that further treatment could cause “injury, disability or death.”
There are countless stories of people having an adverse reaction to the first of two shots — but being encouraged to continue with the second shot anyway. Many of these unfortunates suffered debilitating, lifelong injuries — or death — after the second vaccine.
Meanwhile, instances of doctors or health care workers erring on the side of caution and advising against the second shot for these vulnerable patients are vanishingly rare.
It is increasingly clear that the powerful principles and precepts of the Nuremberg Code have been flouted, even decimated, by those seeking to push the COVID vaccines on every single person on Earth. Foremost among these is the caution against coercion.
Those who resist the anti-American and anti-human idea of a one-size-fits-all medical treatment are freedom-fighters for the obvious and inherent right to choose for themselves. That this is no longer self-evident is deeply alarming.
It is a well-worn aphorism that those who forget their past are condemned to repeat it. Despite mounting evidence of serious adverse events and death, are we doing just that in our increasingly desperate attempts to use coercion to vaccinate absolutely everyone?
Are we nullifying the Nuremberg Code?
WORLD RENOWNED DOCTOR BLOWS LID OFF OF COVID VACCINE
February 15, 2021
A Doctor’s View About the New mRNA Vaccines
By Thomas Siler
It’s important to know both what we know about the new vaccines and what we don’t know.
I’ve practiced for 35 years. I am always honest with my patients, even if conversations are difficult or confrontational. I will also be honest about saying “I don’t know.” This happens when a diagnosis is not readily apparent or when there are limits to the help I can give. With the passage of time, I’ve learned that what we don’t know about medicine outweighs what we do know.
I’ve always been a proponent of older, more established vaccines. However, they are imperfect and, like all medical treatments, can have side effects. Unfortunately, in the conversation about the new COVID-19 vaccines, the tenets of honesty and a willingness to admit ignorance are being compromised.
Operation Warp Speed was remarkable, but it leaves an uncomfortable question: Is it a good thing to rush a vaccine (or medicine) to the public without the usual safeguards? Operation Warp Speed might be a great business objective or military goal, but is it great for a medical treatment?
The pharmaceutical industry, government health authorities, and the media insist the new vaccines are safe and effective. While the initial results are promising, this is not the whole truth. Both honesty and acknowledging ignorance require answering a few questions.
What do we know about the new TYPE of vaccine being given?
Pfizer and Moderna were the first COVID-19 vaccines to be approved. Both use a new technology called mRNA vaccine, which has never been broadly given to a human population to prevent any disease.
Let that sink in for a moment.
All previous vaccines take a weakened virus or a piece of the virus and inject it into humans to induce an immune response sufficient to prevent a disease. Pfizer’s and Moderna’s vaccines inject mRNA, which is a protein code that instructs the body to make a part of COVID-19’s spike protein that will then induce an immune response.
Our bodies daily use our own mRNA to carry instructions from DNA to make various proteins the body uses. While this new vaccine science sounds intriguing, it has never been tried in humans in this scope. It may be a breathtaking scientific advancement heralding a new path for all vaccines. It may also be less effective or have currently unknown side effects.
Is the mRNA vaccine for COVID-19 safe?
So far, the limited study of the vaccines approved for emergency use (one major study for each vaccine approved) has shown some short-term side effects. The vaccine is a two-shot series and side effects were prominent after the second shot. Side effects were more common if the recipient was younger than 65 years old.
Side effects
Pain at the injection site has usually gone away in 4-5 days. The other side effects resolve, on average, in 2-3 days.
Early reports after giving the vaccine have also included allergic reactions ranging from mild to a few cases of anaphylaxis (serious allergic reaction). Allergy may be to mRNA itself or the lipid nanoparticles/PEG vehicle it is housed in. The long-term side effects are not currently known, as the main study length and follow up have only been four months.
Is the mRNA vaccine effective?
In the main study from Pfizer’s vaccine, 8/17,000 patients got symptomatic COVID-19 in the treatment group during the short follow up. In the placebo group, 162/17,000 patients got symptomatic COVID-19 during the study time. There was also a trend towards those getting the vaccine having a less severe disease and needing less hospitalization.
The Moderna study had 30,000 patients split into treatment and placebo arms. In the vaccine group, 11/15,000 patients came down with COVID-19. In the placebo group, 185/15,000 patients came down with COVID-19.
It was hard to ascertain death avoidance in these small studies. However, the two initial studies are favorable and show a 95% efficacy. Now that more information about the studies is known, Peter Doshi, associate editor of the British Medical Journal, wrote an editorial that the true efficacy may be much lower because the study excluded people with COVID-19 symptoms but a negative test and other factors.
How long does immunity last?
This is unknown. Injected mRNA goes away in days, but it is thought that the immune response will be long lasting. Whether patients will need boosters at some point is not known.
What about mutations in the COVID-19 virus? Will the vaccine still work?
Viruses always mutate and scientists following COVID-19 estimate it mutates, on average, twice a month. Most of these mutations are minor and will likely not change the vaccine effectiveness. These mutations also usually do not make the virus more deadly.
What is antibody dependent enhancement?
COVID-19 is in the family of Coronavirus that causes the common cold. The pharmaceutical industry has been trying without success for the last two decades to make a vaccine against the common cold. A safe vaccine against the common cold would make some company a lot of money!
One problem in the animal studies on coronavirus family vaccines was “antibody dependent enhancement.” When animals were inoculated, they developed a robust immune response, which is a good result.
However, when the animals were later exposed to the coronavirus against which they were vaccinated, their immune system went into overdrive, and they developed an overwhelming, fatal immune response called a “cytokine storm.” Fatal cytokine storms also happened to some COVID-19 patients when their infection was severe.
Human responses do not always correlate to animal responses. So far, there have been no signs that humans have a cytokine storm when exposed to COVID-19 after receiving the vaccine. Obviously, this would be catastrophic for any vaccine.
Should we be concerned about other long term side effects from mRNA vaccines?
A concern that deserves mention is the possibility that a cross-reaction and immunity to other parts of the spike protein could cause auto-immune disease or other problems.
A former Pfizer VP, Dr. Michael Yeadon, who has over 30 years of experience in immunology and drug research, filed a Stay of Action petition with the European Medicine Agency (like our FDA) to halt the trials of mRNA vaccines over concerns it might affect sterility in women.
Yeadon is worried that the mRNA vaccine was coded for a region of the spike protein that was similar to Syncytin-1, which is a protein that is essential for the development of the placenta. If a woman’s body makes antibodies to this protein, she could become sterile when vaccinated for COVID-19. This is a theory, not a proven fact, and no one has studied it. Yeadon’s insistence on more studies to make sure this will not happen seems reasonable.
What to make of all these concerns?
Medicine is always about a risk/benefit analysis, subject to the first maxim of “do no harm.” Usually, new medicines or new vaccines are used only after multiple studies show over long periods of time (for vaccines, at least five years) prove they’re safe and better than the older treatments.
While the new mRNA vaccines have good initial results and may be a breakthrough, they should be viewed as experimental and would best be used in high-risk patients (older patients or those with health conditions raising COVID-19 mortality) until we know more. Patients should receive extensive informed consent to understand the risks and benefits. Patients also need to know that if they have a serious complication, Congress already protected the pharmaceutical companies from litigation around emergency vaccines.
The mantra of “safe and effective” is not only incomplete, but it also ignores other pathways out of the pandemic. For healthy people, early outpatient treatments are being developed to treat COVID-19. These would be a safer option than taking an experimental vaccine. Young people (<60 years old) who have very low mortality from COVID-19 should approach getting the new vaccine as if they were consenting to be in an experimental trial of a new vaccine.
Our history shows there are good reasons why new medicines and vaccines are not rushed into widespread use until we have multiple studies and time to assess the safety and efficacy of the new treatments. If the death rate from COVID-19 were much higher, it might make the risks acceptable to try an experimental vaccine. Given that the COVID-19 death rate is a little higher than a bad flu, my opinion is that younger and healthier people need a more rigorous risk/benefit analysis before taking the mRNA vaccines.
It’s Gene Therapy, Not a Vaccine
Portuguese mother, 41, dies two days after taking Pfizer COVID-19 vaccine

PORTO, Portugal, January 6, 2021 (LifeSiteNews) — A Portuguese health care worker and mother-of-two died two days after receiving the Pfizer COVID-19 vaccine.
Sonia Acevedo, 41, was inflicted with “sudden death” on New Year’s Day, following her vaccination which happened on December 30th. An autopsy is expected very soon, the Daily Mail reports.
Acevedo, who worked in pediatrics at the Portuguese Institute of Oncology in Porto, did not experience any immediate side-effects to the vaccine, beyond the “normal” soreness in the area where she received the shot.
Her father, Abilio Acevedo, said she was quite healthy, and “hadn’t had any health problems.”
“She had the Covid-19 vaccine but she didn’t have any symptoms,” he said. “I don't know what happened. I just want answers … I want to know what led to my daughter’s death.”
Ms. Acevedo’s employer, for whom she had worked the past ten years, confirmed she had been vaccinated on December 30 and stated they had not been notified of any “undesirable effect” to the shot in her case. They also expressed their “sincere regret to family and friends in the certainty that this loss is also felt here.”
Her employer added, “The explanation of the cause of death will follow the usual procedures in these circumstances.”
The death of Ms. Acevedo comes in the wake of many concerns regarding these vaccines, which have been rushed through the process of development, testing, approval and now distribution, with a new “messenger RNA” technology, no industry-standard animal trials, nor any sufficient studies on long-term effects.
Indeed, in early December, a former vice president and Chief Scientist at Pfizer, Dr. Michael Yeadon, petitioned for the halting of all testing of coronavirus vaccine candidates in Europe due to the significant safety concerns of a growing number of renowned scientists.
These concerns included, “allergic” and “potentially fatal reactions,” risks that these vaccines may cause infertility in women, result in an increased vulnerability to the virus, and present unacceptable dangers of long-term effects due to a lack of proper testing.
The U.S. Food and Drug Administration also drew up a document this fall listing the possible side-effects from a COVID-19 vaccine, including strokes, encephalitis, auto-immune disease, birth defects, Kawasaki disease and death.
Present reports reveal that hundreds of individuals injected with these vaccines have been admitted to the hospital while high rates of health care workers continue refusing to receive them.
RELATED
Former Pfizer VP: ‘No need for vaccines,’ ‘the pandemic is effectively over’
FDA: Death, heart attacks, stroke, blood disorders all possible side effects of COVID vaccine
Nurses, hospital staff refuse to take COVID vaccine in large numbers
Research scientist warns coronavirus vaccines have ‘terrible safety record’ historically
Doctor calls Fauci’s demand for mass COVID vaccinations ‘utter nonsense’
COVID-19 ‘warp speed’ vaccines likely not safe and not needed, medical expert says
Pfizer COVID jab warning: No breastfeeding, avoid pregnancy for 2 months, unknown fertility impacts
FDA: Death, heart attacks, stroke, blood disorders all possible side effects of COVID vaccin
5 questions about the coronavirus vaccine that should scare everyone

December 5, 2020 (LifeSiteNews) — The United Kingdom has announced its approval of the Pfizer coronavirus vaccine. The U.S. is expected to approve a vaccine within weeks, as well.
The U.K.’s government-produced safety instructions indicate that the vaccine should not be used by pregnant or breastfeeding mothers, that it is unknown what effect the COVID-19 mRNA vaccine will have on fertility, and “women of childbearing age should be advised to avoid pregnancy for at least 2 months after their second dose.”
In light of the fact that despots around the world have suggested, some using stronger language than others, that their citizenries will be forcibly vaccinated, or that there should or will be penalties for not receiving the vaccine, one wonders:
- What happens if a woman receives the vaccine and does become pregnant within two months of receiving it? Will she be pressured to abort her child? What impact would such a vaccine have on the baby?
- Will “women of childbearing age” be pressured — or even forced — to go on birth control or forgo pregnancy in order to receive the vaccine, which has been touted as the all-important key to “going back to normal”? In what world would it be considered just or fair to tell women they mustn’t become pregnant so they can receive an optional medical intervention — which has no shortage of side effects and risks — for a disease the vast majority of people survive?
- Will new mothers be pressured to forgo breastfeeding so they can be vaccinated?
- Will Catholic bishops tell women they ought to forgo pregnancy so they can be vaccinated? The Catholic bishops of California say they are committed “to promoting and encouraging COVID-19 vaccinations in the communities we serve.” If they will tell women to forgo childbearing, at least temporarily, so they can receive the shot, how will that be squared with Church teaching that children are the primary end of marriage and contraception is intrinsically evil?
- According to the U.K. government’s guidelines, which will presumably be similar to Pfizer vaccine guidances in other countries, pregnant women shouldn’t receive the shot. Will unvaccinated pregnant women be discriminated against because of this, and denied access to airlines and other spaces?
The fact that these questions even need to be asked is chilling (not as chilling as the vaccine itself, which bizarrely has to be stored at -70°C — colder than Antarctia).
The future of the human race, civil society, and very basic freedoms are at stake here. The time to ask these questions is now. It’s not too late — but it will be soon.
Covid vaccines – like apples and oranges
22 July 2020
Content Sections
By Robert Verkerk PhD, founder, executive & scientific director
The vaccine race is well and truly on as you’ll see from the Milken Institute’s Covid-19 Treatment and Vaccine tracker. But it could just as easily be described as a vaccine war – with the US having accused China of cyber-based espionage for stealing secrets from US vaccine companies that might give Chinese efforts an advantage. Hidden away in the 130-page supplementary appendix published in The Lancet alongside the Oxford/AstraZeneca Phase 1/2 trial, the British team have definitely got it in for the US team at Moderna/NIAID, stating: “Subunit vaccines [like Moderna’s] usually require the use of adjuvants and whilst DNA and RNA vaccines can offer manufacturing advantages, they are often poorly immunogenic requiring multiple doses, which is highly undesirable in the context of a pandemic.” [see Section 3.5 of supplementary appendix, p. 72]
Cooperation? Competition? Or war?
So much for the $18 billion global cooperation effort on vaccines, supposedly led by the World Health Organization (WHO) and Gates’ brainchild, the Coalition for Epidemic Preparedness Innovations (CEPI), that Gates threw nearly $100 million at in 2017.
The idea, agreed at an extraordinary virtual summit of the G20 on the 26th March, was that there would be no ‘profiteering’ over vaccines. The US Food and Drug Administration (FDA) has also widely claimed its commitment to full transparency, including around clinical trials and data. Recognising reports that the US President has pressurised the FDA to cut corners on vaccine development and deployment, a US House of Representatives Oversight Committee has demanded transparency to allow independent scientific review of vaccine safety and effectiveness.
As we start to digest the flurry of Phase 1 and 2 clinical trial reports coming out in some of the world’s most prestigious journals, we’ve been increasingly shocked. It’s a million miles from the kind of transparency we believe is in the public interest that we outline in our Vaccine Transparency Manifesto.
Which data are hidden?
Here are our three top concerns:
-
Data disclosure is definitely not transparent. Even in the case of the Oxford/AstraZeneca vaccine trial with its 130 pages of supplementary data, there are huge holes in the data – including about which subjects suffered which adverse reactions, and no breakdown of the adverse reaction data for the biggest groups tested.
-
The trial designs are not standardised. Take the two non-replicating viral vector vaccines, the Chinese CanSino Biologics/Beijing Institute for Technology one and the Oxford/AstraZeneca one. Both trials are published in the same journal, The Lancet, one of the world’s most prestigious medical journals. The CanSino one is double blind, the Oxford one single blind. Adverse events for the two trials cover entirely different time periods: 14 days for the CanSino vaccine, and solicited adverse event reports in the first 7 days following vaccination in the case of the Oxford vaccine and 28 days for unsolicited reactions. Apples and oranges.
-
Prestigious journals have dropped their standards for research reporting. Tell us how The Lancet allows the Methods in the CanSino trial to mention that the control involves only the adjuvant, yet fails to mention what it is – even broadly speaking, assuming some might argue for confidentiality to protect intellectual property. Wasn’t this all meant to be for the greater good, and open book? Why wasn’t the detailed data for the CanSino trial published in the The Lancet, when in the same journal, the Oxford trial included 130 pages of supplementary data? But in this, why is the detailed breakdown of adverse event reactions for the most important Group 2/4 plain missing? Why is it we can’t work out which subjects suffered from multiple adverse reactions?
Many vaccine candidates are already in manufacture preparing for roll-out in early 2021 if given green light by regulators.
The contenders
The World Health Organization (WHO) tells us there are 24 candidate vaccines currently being evaluated clinically in humans. In case you thought the pipeline might be a little light, the WHO assures us there are a further 142 candidate vaccines in pre-clinical evaluation.
The contenders for commercial vaccines are publishing their preliminary data, and there’s huge political and public pressure for one or more of these to be rolled out by the end of this or early next. Among those at the front of the pack are the following 5:
-
Moderna/National Institute of Allergy mRNA-1273. We reported on this one last week. The work is being coordinated by Dr Anthony Fauci, head of the National Institute of Health’s National Institute of Allergy and Infectious Diseases. It’s a genetically engineered mRNA vaccine that’s delivered using a lipid nano particle (LNP) encapsulation system. The mRNA is encoded to instruct the muscle cells of the vaccinated person to express a stabilised form of the virus spike protein. It effectively turns your muscles into a vaccine factory and from there it triggers a humoral (antibody) and cell-mediated (T-cell) response. As we reported, even in a very small trial of just 45 people, it produced significant and severe side effects in some and moderately severe adverse reactions in 80% of those vaccinated.
-
Oxford-AstraZeneca vaccine. This is a non-replicating viral vector vaccine. It’s based on a synthetic, genetically engineered chimpanzee adenovirus. Once injected, the synthetic code causes the vaccinated person’s body to produce the coronavirus spike protein. That in turn triggers the immune system, both the humoral (antibody) and cell-mediated (T-cell) side. A booster dose is required to trigger sufficient immune response to be potentially useful in the real world. The team at the Jenner Institute had already been working on finding relatively innocuous viruses that could act as vectors for Ebola, so they could quickly redirect their efforts to Covid-19 in January. Some argue that the Oxford vaccine is presently leading the vaccine race.
While the trial results were promising from an immunogenicity viewpoint, the median age of the healthy participants was only 35, so we still don’t know much about how it might work on older people, including with comorbidities, who suffer the most serious cases of disease. Nor do we know much about how it affects those of non-white ethnicity, given 91% of subjects were white. The vaccine is non-adjuvanted which is a plus in terms of side effects, but the vaccine still showed more adverse reactions than the comparators, either Pfizer’s Nimenrix or GSK’s Menveo vaccines, both targeting meningitis (MenACWY). We also cannot deduce from the non-transparent data which subjects suffered different combinations of adverse events, and detailed adverse event data relates only to the initial Group 1 (n= 88) trial that also tested the effects of paracetamol. Surprisingly there were no comprehensive data on the Group 2/4 in which 489 volunteers were vaccinated with the genetically engineered chimpanzee virus vector. In short, the data are insufficient to allow independent evaluation of safety.
-
CanSino Biologics/Beijing Institute of Technology. Like the Oxford-AstraZeneca vaccine, this one is also based on a non-replicating viral vector vaccine. This time, the vector is a weakened, replication-defective human adenovirus (type 5) that causes the ‘common cold’ and does contain an adjuvant. Like Moderna, CanSino has never produced a vaccine before, but it’s very well funded and has the might of the Chinese government behind it. The project, as well as executives at CanSino, have strong ties with Canada. We know the vaccine is adjuvanted because the recently published Lancet paper with preliminary results of its double, not single, blind trials, tells us this much in its methods, the control being the same as the treatment (i.e. just so-called excipients) minus the genetically engineered sub-unit of the viral vector. But incredibly in our view, the Lancet paper omits to tell us what the adjuvant is. Is it an aluminium salt? Possibly, but we don’t know. The immune response looks good at face value, but it’s impossible to compare with other vaccines as no assessments are comparable. As far as adverse events are concerned, 9% suffered severe ones at the highest dose, 1% at the lower dose. Given that immunogenicity was comparable, they’re now going for the lower dose in the Phase 3 trial. Putting that in perspective, assuming a country the size of the UK, even the lower dose, if exposed to 70% of the UK population, would elicit 466,550 severe adverse events based on these preliminary results. That’s over one and a half times more than have been confirmed infected by Covid-19 in the UK to-date.
-
Pfizer-BioNtech’s BNT162b vaccine. Don’t discount this one – using the same lipid nano particle (LNP) platform as Moderna, with the world’s largest pharma company, Pfizer, in the mix for manufacturing and roll-out. Initial results are cited as promising in terms of immunogenicity and "lack of adverse events. The UK has ordered 30 million doses of one of two of the mRNA vaccines, whichever works best. But that’s dwarfed by a patriotic US order of 600 million doses.
-
Zydus Cadila - ZyCoV-D vaccine. Another candidate we should all keep our eyes on is the all-Indian vaccine project, based on a plasmid DNA vaccine. Details are sketchy, but apparently preclinicals yielded a strong immune response.
Transparency, or no trust
We’re moving rapidly towards a significant crossroads - one we can't afford to be sleeping at the wheel at when we arrive. Those who believe (and right now, it’s about belief and not scientific evidence) that vaccines are our only way out of this pandemic and into some semblance of normality, better soon realise that transparency is going to be a prerequisite. Many who have been denigrated as ‘anti-vaxxers’ or vaccine hesitants are simply those wanting more information, those who are concerned about the abominable lack of transparency around vaccine development and trials, or are parents or family members of those who have been vaccine-injured.
Hidden by a PR-machine intent on preparing people to roll up their sleeves is another critically important issue no one seems to be talking about. Where was the public debate on genetically engineered vaccines? Europeans have long been opposed to consuming genetically engineered foods – and many US states fought hard to force companies to label products containing genetically modified ingredients, albeit often unsuccessfully given the might of the pro-GMO lobby.
But at least foods are filtered through the digestive tract, including our very sophisticated intestinal mucosa and gut microbiome. Vaccines, including any adjuvants, other excipients and any free-loading contaminants bypass this sensing system. Many of the genetically engineered vaccines now heading the vaccine race do things we recently reserved for science fiction. They get our bodies to become the vaccine factories, having received information to do this from instructions issued by synthetic genetically coded material.
If you’re OK with all of this, that’s fine. But we believe you should be told and given all available information about the known risks and benefits, as well as about the composition of the medical treatment you’re being subjected to - before it’s given. That’s what medical consent is about - and it’s written into the rule book of every supposedly civilised nation, yet so often flouted in the case of vaccination.
We owe it to future generations to push the authorities to ensure we’re all provided with all the information we need to make an informed choice prior to being exposed to such an unproven medical procedure that breaks all the laws of nature that have preceded us for around four and half billion years. This issue will come to a head if the G20 countries get to produce their desired new, chip-enabled, machine-readable immunisation record that will be as important as your passport if you want to move from your country of residence.
Take action NOW!
Find out more about our Vaccine Transparency Manifesto and tell your elected representative why it’s so important for all of our futures by downloading the UK or international flyer below.
>> Download UK flyer as PDF
>>> Download international flyer as PDF
Which COVID-19 vaccines will be derived from aborted children’s cell lines?
There are now more than 120 COVID-19 vaccine candidates in development.
Navigating the ethics of using them will be a challenge.
Thu Jun 11, 2020 - 9:33 am EST
By Jonathan Abbamonte
June 11, 2020 (Population Research Institute) — The race is on to find a vaccine for COVID-19. The good news is that many of the world’s largest vaccine companies are developing promising vaccine candidates using ethically-derived cells. The bad news is that many of the leading vaccine candidates for the 2019 novel coronavirus (SARS-CoV2) are being developed using fetal cell lines that were originally derived from the tissues of aborted babies in the 1970s and 80s.
With more than 6.2 million reported cases so far and more than 375,000 deaths worldwide, the burden of disease from the 2019 novel coronavirus continues to mount. And so does the urgency to find a cure. From big pharma to small biotech companies and universities, researchers have been pushing out dozens of vaccine candidates and have fast-tracked promising vaccine candidates to clinical trials in record time. Pharmaceutical companies are sprinting to have a vaccine ready by the end of the year or by early 2021.
According to a tracker from the World Health Organization, there are now more than 120 vaccine candidates in development. Of these, 10 vaccine candidates have already advanced to clinical trials to test the vaccine candidate’s safety and efficacy. Several more candidates are expected to begin clinical trials before the end of the year.
Fetal Stem Cells Being Used
Several COVID-19 vaccine frontrunners, including those being developed by Moderna, Oxford University/AstraZeneca, CanSino Biologics/Beijing Institute of Biotechnology, and Inovio Pharmaceuticals, are using a human fetal kidney cell line called HEK-293 to develop their trial vaccines. HEK-293 was originally derived from kidney tissue taken from a baby girl who was aborted in the Netherlands in 1972 and later developed into a cell line in a lab in 1973.
Additionally, Janssen, the pharmaceutical division of consumer product giant Johnson & Johnson, is using the human fetal cell line PER.C6 to develop its vaccine. The PER.C6 fetal cell line was derived from retinal tissue taken from an 18-week-old baby boy who was aborted in the Netherlands in 1985 and later converted into a fetal cell line in 1995.
The U.S. government has made grants totaling nearly $2 billion in support of the development of COVID-19 vaccines using fetal cell lines. Most of this funding has been awarded through the Biomedical Advanced Research and Development Authority (BARDA), a division within the U.S. Department of Health and Human Services (HHS).
BARDA has awarded a $1.2 billion grant for AstraZeneca to fund research for the trial vaccine it is jointly developing with Oxford University. BARDA has also made grants for up to $483 million for Moderna’s vaccine and $456 million for Janssen Research and Development, LLC of Johnson & Johnson. Inovio has also received an unspecified grant for developing its vaccine candidate from the Defense Advanced Research Projects Agency (DARPA) at the Department of Defense.
On June 1st, BARDA issued a $628 million task order under a preexisting government contract with Emergent BioSolutions Inc. to accelerate development and manufacturing capacity for COVID-19 vaccines and drug treatments. Emergent BioSolutions is currently working with Janssen of Johnson & Johnson to manufacture their trial vaccines. BARDA’s funding for Emergent, however, was not awarded specifically for scaling up production of J&J’s vaccine candidate.
Worse still, the U.S. Centers for Disease Control and Prevention (CDC) has been producing samples of the SARS-CoV2 virus for biotech and pharmaceutical companies to use for vaccine research using fetal HEK-293T cells (a decedent cell line of HEK-293).
Moderna is also receiving substantial research assistance for its COVID-19 vaccine from the National Institute of Allergy and Infectious Diseases (NIAID) which helped develop the vaccine and conduct clinical trials. NIAID is a division of the National Institutes of Health (NIH) led by Dr. Anthony Fauci.
Ethically Produced COVID-19 Vaccines in the Pipeline
While many COVID-19 vaccines are being developed with fetal cell lines, a number of promising vaccine candidates, such as those being developed by Novavax, Sanofi Pasteur, GlaxoSmithKline (GSK), and Sinovac, are using ethically derived cell lines.
Of particular note, rival pharmaceutical giants Sanofi Pasteur and GSK have teamed up in an unprecedented partnership to jointly develop a vaccine for SARS-CoV2. Sanofi Pasteur will be bringing to the table an ethically produced antigen for the vaccine and GSK will be contributing an adjuvant — an immune response booster that improves the effectiveness of a vaccine.
U.K.-based GSK and France-based Sanofi are the world’s #1 and #3 largest vaccine producers respectively by total revenue in 2017 according to FiercePharma.
A vaccine being developed by Maryland-based Novavax is using an ethically derived invertebrate cell line Sf9 to produce protein nanoparticle antigens that make its vaccine work.
In animal studies, Novavax’s candidate demonstrated that the vaccine produces antibodies to the SARS-CoV2 spike protein and produces neutralizing antibodies capable of isolating and destroying the SARS-CoV2 virus. Novavax’s vaccine has already been approved for a fast-tracked phase I/II stage clinical trial. Results for the vaccine candidate’s safety profile and immunogenicity (the ability to induce an effective immune response in the body) are expected by July.
Sinovac, a China-based biotech company, is also working on an ethically derived vaccine candidate called PiCoVacc. PiCoVacc uses a purified inactivated SARS-CoV2 as an antigen. Sinovac’s antigen is ethically grown in Vero (monkey kidney) cells. Sinovac’s vaccine is currently undergoing expedited phase I/II clinical trials.
Pharmaceutical giant Merck also recently jumped into the COVID-19 vaccine race with an announcement on May 26th that the company will be pursuing 3 vaccine candidates. Merck was the first company to develop a proven vaccine for Ebola. Merck’s Ebola vaccine was granted regulatory approval by the FDA last December.
As of the writing of this article, it is still too early to tell whether Merck’s COVID-19 vaccines will use fetal cell lines or ethically derived cells.
But one of Merck’s vaccine candidates for COVID-19 being developed in partnership with the International AIDS Vaccine Initiative (IAVI) will be utilizing the same platform Merck used in successfully developing its Ebola vaccine (V290). The company’s Ebola vaccine is manufactured using a cell line ethically derived from the kidney cells of an African green monkey.
Another vaccine candidate being developed by Merck through Themis, a biotech company recently acquired by Merck, is seeking to use the live measles vaccine as a viral vector. Merck’s measles vaccine is produced using chicken egg [Note: Merck’s MMR vaccine (measles-mumps-rubella) is manufactured using human fetal cell line WI-38 which was derived from the lung cells of an aborted baby].
How Do Vaccines Work?
Cell lines are often used in vaccine production to grow viral proteins that make the vaccine work.
Vaccines produce immunity by training immune cells to fight off infection by exposing them to weakened or dead viruses or an isolated protein from the virus (or a synthetic look-alike). Providing immune cells the chance to fight off weakened viruses or viral fragments prepares the body to identify and neutralize the virus if encountered in the future.
Weakened viruses, inactivated viruses, and viral proteins used in a vaccine to produce immunity are called antigens. Antigens are any protein or molecule that triggers an immune response in the body, causing immune cells to produce antibodies. Antibodies are proteins the body’s immune cells produce to bind with and tag viruses and harmful bacteria with markers that help the immune system identify and destroy pathogens.
Traditionally, vaccines are manufactured by growing antigens in animal, plant, or fungi cells or tissues such as embryonated chicken eggs, yeast, or monkey kidney cells. After the antigens have been grown in these cells, the antigens are harvested, purified, and then added to a solution that is later injected or ingested as a vaccine.
Sometimes, however, vaccine manufacturers will use human fetal cells instead of animal cells to grow the antigens for their vaccines.
Several COVID-19 vaccines under development, such as those being developed by Oxford University, CanSino Biologics, and Johnson & Johnson, are utilizing a technology known as “non-replicating viral vector” vaccines.
Unlike traditional vaccines which involve injecting antigens into the body that were previously grown in chicken eggs or Petri dishes, viral vector vaccines grow the antigens in a person’s own cells.
In viral vector vaccines, a segment of DNA from the SARS-CoV2 virus is spliced into the genome of a benign carrier virus. The viral vector is also genetically modified to prevent the virus from replicating. When injected into the body, the viral vectors carry coronavirus DNA to the body’s cells that provide the cells with instructions on how to make antigens.
In order to make these vaccines, vaccine manufacturers must grow a sufficient number of these genetically modified viral vectors to induce immunity. Viral vector vaccines being developed by Oxford University, CanSino Biologics, and Johnson & Johnson are currently using fetal cell lines from aborted babies to grow their viral vectors.
A number of COVID-19 vaccine candidates under development are utilizing completely new vaccine platforms that require no cells at all. Several biotech and pharmaceutical companies are racing to develop vaccines that contain no viral proteins at all but only messenger RNA (mRNA) or DNA plasmids that provide the body’s cells with instructions on how to produce antigens.
Although no mRNA or DNA plasmid vaccine has yet received regulatory approval for normal use, the technology is promising. Experimental mRNA vaccines in recent years have shown promising results in clinical trials and in animal studies. mRNA vaccines could have distinct advantages over traditional vaccines because they can be developed much faster, cheaper, with a higher potency, and they can even be created without samples of the pathogen. The genome sequence is all that is needed to create these vaccines, slashing the amount of time it takes to produce a vaccine.
Additionally, mRNA- and DNA-based vaccines have the benefit that no fetal cells (or any cells for that matter) are needed to produce them.
Moderna is developing a mRNA COVID-19 vaccine candidate (mRNA-1273) in collaboration with NIAID. Meanwhile, Inovio Pharmaceuticals is developing a DNA plasmid vaccine for SARS-CoV2 (INO-4800).
Despite the fact that no fetal cell lines are needed to produce DNA plasmid vaccines, Inovio has chosen to test the immunogenicity and efficacy of its vaccine candidate using human fetal kidney cells (HEK-293T), sadly tainting what could otherwise have been a promising vaccine candidate.
As for Moderna’s vaccine, NIAID claims to have helped Moderna develop mRNA-1273.
In February, NIAID scientists working in collaboration with researchers at the University of Texas at Austin (UT) successfully identified and isolated the SARS-CoV2 spike (S) protein and its receptor binding domain using previous research it had on similar coronaviruses such as those which cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). However, the scientists in this study used human fetal kidney cells (HEK-293) to culture the SARS-CoV2 virus before isolating the S protein. The results from this study were later published in the peer-reviewed journal Science.
In a news release on February 19th, NIAID claimed that the study “supports NIAID’s approach to a gene-based vaccine for COVID-19.” In the same new release, NIAID also mentioned that it was working with Moderna to produce a mRNA vaccine, presumably using the findings from its study using HEK-293 cells to help identify the genetic sequence for mRNA vaccine.
Moral and Ethical Issues with Using Fetal Cell Lines to Develop Vaccines
The use of fetal cell lines for vaccine production has long stirred significant controversy among ethicists and people of faith, particularly among Catholics and Protestants that have deep religious and moral objections against the use of cell lines that were originally derived from aborted babies.
The Catholic Church has long vocally opposed the development of vaccines using unethically derived fetal cell lines. The Congregation for the Doctrine of the Faith’s 2008 Instruction Dignitas Personae states that the use of fetal cell lines for developing vaccines “gives rise to various ethical problems with regard to cooperation in evil and with regard to scandal” and that “everyone has the duty to make known their disagreement and to ask that their healthcare system make other types of vaccines available.”
Although it is not widely known or clearly disclosed by pharmaceutical companies, fetal cell lines have been used for decades in the development and manufacture of many widely used vaccinations including measles/mumps/rubella (MMR), rubella, varicella (chickenpox), polio, hepatitis A, rabies, and shingles. For some vaccines, such as MMR, chickenpox, and hepatitis A, no ethically produced alternatives exist in the U.S.
Why Are Fetal Cells Ever Used to Manufacture Vaccines?
Although alternative ethically derived cell lines exist for vaccine development, pharmaceutical companies often prefer to use fetal cell lines because the characteristics of fetal cell lines are well known and because they do not contain significant contaminating viruses or bacteria that are often found in cells derived from animals.
For example, the polio vaccine in the mid-20th century was once manufactured using primary cell cultures harvested from the kidneys of monkeys. However, it was later discovered that the vaccines were often contaminated with a common monkey virus known as Simian Virus 40 (SV40). Although SV40 is harmless to humans, the incident alarmed vaccine manufacturers. Since then, vaccine developers have relied more heavily on cell lines rather than cell cultures taken from live animals.
To provide a more contemporary example, the FDA is currently investigating whether there is any potential from harm in using primate cells for vaccines and biologics. Simian foamy viruses (SFV) are widespread among non-human primates and it is known that these viruses are sometimes able to cause infection in humans. Although no one is ever known to have become sick as a result of SFV, the FDA is investigating whether there could be long-term effects due to this.
With cell lines, it is possible for researchers to know the characteristics and inherent flaws in the cells they are working with. Cell lines have been rigorously inspected by scientists across the industry for contaminating virus DNA or genetic mutations, whereas tissues taken directly from live animals may have unknown contaminants that may potentially be harmful to humans.
But why use fetal cell lines instead of animal cell lines or embryonated chicken eggs which have an excellent track record and have been used for decades to manufacture vaccines? Many vaccines, including vaccines for the seasonal flu, are grown in embryonated chicken eggs.
However, there is a potential for supply issues when using chicken eggs to manufacture vaccines. If, for instance, there were a widespread outbreak affecting chickens that causes the supply of eggs to suddenly drop, it could impact the ability for manufacturers to make vaccines quickly. The supply issue could especially present problems in the event of a pandemic such as the current COVID-19 pandemic where the ability to produce hundreds of millions of vaccines quickly is paramount.
More significantly however, some viruses, such as chickenpox for instance, do not grow well in animal cells. In such cases, there are few other options available other than using human cell lines for vaccine production.
Researchers also often prefer to use human cells for experiments testing the effect of drugs or vaccines because they more closely resemble how a drug will work in humans.
But if human cells are better for manufacturing certain vaccines, why are fetal cell lines derived from aborted babies used instead of ethically derived adult cells?
Fetal cells are often preferred to adult cells because there are a limited number of splittings (passages) cells can undergo before they age and eventually die off. Fetal cell lines can be put through more passages than adult cells would and they are less prone to cell aging and senescence (when cells in a culture no longer divide and start dying off). Fetal cells are also less likely to be contaminated with human viruses or with genetic mutations or alterations that naturally occur as cells age.
Certain fetal cell lines such as PER.C6 are uniquely designed for manufacturing non-replicating viral vector vaccines. The viral vectors are genetically modified to prevent replication in the human body by deleting a portion of the viral vector’s genome. PER.C6 cells are designed to fill in this deleted genome gap. In this way, vaccine manufacturers can replicate viral vectors for their vaccines in the lab but such viruses are incapable of producing an ongoing infection in the body.
However, there is no need to derive cell lines from aborted babies. Human cell lines for vaccines could easily be produced in an ethical manner if they are derived from adult cells. Cell lines could be derived from tissue discarded during surgery or from organs donated after death. If fetal cells are needed, there are thousands of stillbirths and neonatal deaths in the U.S. every year. There is no reason why cell cultures cannot be derived from tissue donated from prematurely born infants that, despite the best medical technology, aren’t able to survive and die of natural causes while in a hospital setting. In these scenarios, developing a cell line would be no different from an ethical perspective than donating organs. In such context, however, it is imperative that any tissues obtained from naturally deceased neonates be strictly done in an ethical manner that respects the fundamental right to life of the child and the deceased child's bodily integrity. The procurement of tissue from the deceased could present a whole new set of ethical problems, particularly if the primary right to life of the donor is not sufficiently respected.
There is also the possibility that cell lines could be developed using cells ethically derived from human umbilical cord, cord blood, or placental tissue—tissues and organs that hospitals routinely discard as medical waste.
And ethical alternatives for human cell lines specially designed for producing viral vector vaccines may soon be available to vaccine manufacturers.
The John Paul II Medical Research Institute in collaboration with Cellular Engineering Technologies (CET) is currently in the process of developing an ethically-derived adult human cell line specially designed for growing viral vectors for vaccines that could replace ethically-fraught cell lines like HEK-293 and PER.C6.
But even so, it is not necessary for viral vector vaccines to be manufactured using fetal cell lines. Merck’s Ebola vaccine, for instance, is a viral vector vaccine that is grown in monkey kidney cells.
Immortalized adult human cell lines that were ethically derived from the cancer cells of cancer patients have also been available to researchers for decades. Immortalized human cancerous cell lines have some of the benefits of fetal cell lines in that they are high passage cells (in fact cancerous cell lines divide infinitely). However, the genetic mutations in these cell lines often change too much and there is concern that these cells could be contaminated with oncogenic viruses (i.e. viruses that induce the formation of cancerous tumors). There is fear that DNA from oncogenic viruses could find their way into vaccines if these cell lines are used for manufacturing vaccines.
However, fetal cell lines such HEK-293 and PER.C6 are also tumorigenic. There is concern that these cell lines too could be infected with oncogenic viruses or oncogenic DNA.
Although rigorous purification processes are used when manufacturing vaccines, purification is an arduous process and it is practically impossible to filter out all contaminants. The FDA is currently researching tests and methods to better determine whether certain cell lines are safe enough for vaccine production.
For the current ongoing COVID-19 pandemic, there is no reason for vaccines to be developed using unethically-derived fetal cell lines. Many of the world’s largest vaccine companies, including Sanofi, GSK, Merck, and Novavax, have demonstrated that it is possible to develop promising vaccine candidates using ethically-derived cells such as Vero, Sf9, and perhaps even embryonated chicken egg. Ethical alternatives exist.
If pharmaceutical companies are not willing to use ethical alternatives, then they must be required to.
Published with permission from Population Research Institute.
The 10-point vaccine transparency approach
29 April 2020
ANH together with the British Society for Ecological Medicine calls on UK government to heed vaccine transparency
Content Sections
We’re told repeatedly by our governments that we’ll only be allowed to emerge from various degrees of restriction to our freedoms once a vaccine is ready. That might take 12 to 18 months. We’re being given the impression it’s a straightforward process, that’s why it can be fast-tracked at a rate that surpasses any other vaccine ever produced. Hindsight’s a fine thing, but surely we need to learn from what went wrong last time round - when vaccines were produced for the last pandemic, the influenza A/H1N1 ‘swine flu’ virus back in 2009/10?
The solution has to be vaccine transparency. And we need to change the narrative from the World Health Organization (WHO)’s ‘vaccine hesitancy’, that the WHO rates as among the 10 greatest threats to global health, to vaccine transparency.
- Find related articles, information and videos in our Covid Zone
So today, in conjunction with our medical doctor colleagues at the British Society for Ecological Medicine, we’ve sent an open letter to Matt Hancock, the UK Secretary of State for Health and Social Care calling on a new public narrative around vaccines. This narrative is about transparency, something that’s been sorely missing through the development and roll-out of a number of recent vaccines.
Download Open Letter to Matt Hancock that includes the 10-point vaccine transparency approach (the full letter can be found in text below)
The taboo that has been created around even debating vaccination is unacceptable in a world that is rushing ahead with the development of global vaccines for Covid-19, often relying on untried or embryonic technology platforms.
Instead of pitching the blame at those citizens who choose to not give their consent for their own or their children’s vaccination, the powers-that-be must recognise their own role in contributing to this situation through the withholding of data and information, as well as inadequate safety testing.
Transparency must occur in multiple areas: clinical trial designs, the results from trials, raw data from trials to allow independent analysis, clarification around vaccine injury payments in the event of no-fault injuries, eligibility criteria for such payments, and, among other things, details of government indemnities, where applicable, for vaccine manufacturers.
The aim is to avoid the mistakes of the past in which sponsorship bias, withholding of data by health authorities, incomplete communication of information to the public and the academic community, among other shortcomings, has led to unnecessary vaccine injury and public distrust of vaccines.
Members of the public or academics who seek answers to questions around vaccine safety have been routinely vilified and labelled ‘anti-vaxxers’ and their communications are censored on social media.
If we are to establish a ‘new normal’, as our politicians seem intent to do, this approach is not tenable. Lack of transparency around the development and testing of Covid-19 vaccines will lead to further divisions in communities, at a time when division will only exacerbate the challenges facing societies since the pandemic arose. It will give governments more reason to deny citizens fundamental human rights and freedoms, as well as increase the risk of martial law being imposed.
The narrative around vaccines must fundamentally change. We must transition away from coercive public policy driven by vaccine protagonists that projects a view of the unassailable safety and effectiveness of vaccines. Doing so only misleads the public over the quality and certainty of the science on which mass vaccination programmes are justified, and denies the public the information needed for properly informed consent.
We call on our friends and colleagues in other parts of the world to also pressure their governments to heed vaccine transparency, using whatever parts of our letter to Matt Hancock that may be relevant.
Open letter to the UK Secretary of State for Health and Social Care, Matt Hancock MP
Open letter to the Rt Hon Matt Hancock MP [By email and hard copy]
The Rt Hon Matt Hancock MP
Secretary of State for Health and Social Care
House of Commons
London,
SW1A 0AA
29 April 2020
Dear Secretary of State
RE THE CRITICAL NEED FOR TRANSPARENCY AROUND COVID-19 VACCINES
As a non-profit organisation representing diverse interests in natural and sustainable health, and a medical association of doctors who practice ecological (including nutritional and environmental) medicine, we hereby request that the Department of Health, the Joint Committee on Vaccination and Immunisation (JVCI), the UK Vaccine Network, Public Health England and the Medicines and Healthcare products Regulatory Agency (MHRA) maintain a policy of full transparency around the development, testing and roll-out of vaccines targeting Covid-19.
The UK Government, other governments and health authorities, including the World Health Organization, have repeatedly made clear concerns over vaccine hesitancy and the potential impact on public health.
Two major drivers of vaccine hesitancy include:
- Low levels of trust in the medical science behind vaccination safety and effectiveness, pharmaceutical companies who produce these vaccines, and government health agencies who promote vaccination (Xu et al, Health Comm. 2020; Apr 19: 1-14). Trust is readily eroded by misleading claims issued by health authorities which consistently refer to vaccines as ‘safe’ when it is clear that adverse events occur at varying, albeit low, frequencies. To-date, in the UK, around 1000 claims have been paid out to those who have been severely disabled (from over 6,000 claims) after establishing proof of causation through the Vaccine Damage Payment Act 1979. Furthermore, public trust in a pandemic vaccine will have been adversely affected by claims that vaccines targeting the influenza A/H1N1 ‘swine flu’ pandemic of 2009 had been “thoroughly tested” when this was more recently found to be false (Doshi P. BMJ 2018; 362: k3948);
- Insufficient communication of relevant information, including trial designs and results by health authorities and vaccine manufacturers. Such inadequacies have been revealed around HPV vaccine trials (Doshi et al. BMJ Evid Based Med. 2020; pii: bmjebm-2019-111331) as part of the Restoring Invisible and Abandoned Trials initiative (RIAT) and in retrospective analysis of information and events surrounding the roll out of vaccines during the last pandemic (influenza A/H1N1, ‘swine flu’) in 2009 (Stephen W. BMJ2018; 362: k3948).
Health authorities, as vaccine protagonists, must therefore take some responsibility for their role in creating an environment that fosters distrust and hesitancy over vaccination rather than always blaming citizens or scientists for being irrational when they express concerns about vaccine testing or safety. Coercive public policy on vaccination, coupled with the categorisation of comments by citizens, doctors and others that question vaccine safety as ‘fake news’, which then often leads to censorship, are therefore counter-productive.
Informed risk/utility decisions around mass vaccination require increasing public engagement (Williamson & Glaab. BMC Med Ethics. 2018; 19(1): 84) and benefit from clear disclosure of sponsorship bias and the capacity for re-analysis of raw data by independent researchers (Jefferson T. J R Soc Med. 2020; 113(4): 148-157). Full disclosure of results from clinical trials, including provision of raw data, is vital given data on fast-tracked vaccines will inevitably be uncertain and incomplete to some degree. It is important that the extent of such shortcomings are communicated to the public.
It is therefore in the public interest to ensure that all relevant data that could feed into properly informed decisions are placed in the academic and public domains. Public confidence in vaccination can only be re-established if there is much greater transparency and sharing of data than has been the case historically (Godlee F. BMJ 2018; 363: k4152). This is more relevant than ever with the prospect of Covid-19 vaccines, given their unprecedented rate of development.
Key areas for vaccine transparency
Having consulted with medical doctors, other health professionals, research scientists, lawyers and citizens in our various networks, we consider it imperative that the following information is released for public scrutiny prior to commercial release of any Covid-19 vaccines:
- Full disclosure of all raw data from safety studies of commercial Covid-19 vaccines. Disclosure of raw data allows independent researchers to analyse data and draw conclusions independently of health authorities, regulators and vaccine manufacturers. Such transparency and data sharing are essential if the aim is to establish confidence in mass immunisation using a novel vaccine developed in a fraction of the time typical of previous vaccines;
- Transparency in relation to safety and efficacy studies. Safety studies for any vaccine that is fast-tracked (6-18 months) prior to approval will be compromised as compared with those for which more time (several years) has been allowed for safety studies and regulatory approval. If the Government is planning to encourage vaccination, it is crucial that it is clear about the limitations in safety and efficacy studies supporting public roll-out as compared with those required for normal licensing of vaccines. Without such knowledge, it is neither possible for citizens to balance risk versus utility, nor can they determine “…if the safety of the product is not such as persons generally are entitled to expect” (Consumer Protection Act 1987);
- Transparency over the type of platform used for commercial vaccines. Currently there are several different platforms being investigated for candidate vaccines for Covid-19 and it appears that the most likely (and well funded) options involve platforms that have never been previously used on a global scale (Amanat & Krammer. Immunity. 2020; 52(4): 583-589). It is imperative that there is clear communication to the public over the nature of the platform(s) being used for Covid-19 vaccines prior to their commercial release, as well as the extent of their previous use, if relevant, for pre-existing commercial vaccines;
- Conduct and transparency of studies to elucidate any risks associated with adjuvants as distinct from antigens. Given that commercial vaccines for Covid-19 are likely to be adjuvanted, it is essential that the safety of the adjuvanted vaccines are compared with non-adjuvanted vaccines and saline controls. Adjuvants may trigger specific side effects in susceptible individuals, which may include those with underlying conditions, including autoimmune diseases (e.g. Watad A, et al. Front Endocrinol (Lausanne). 2017; 7: 150);
- Transparency in relation to vaccine composition. There is a significant public lack of confidence in the purity and composition of vaccines. It is essential that the detailed composition of Covid-19 vaccines are declared, this going beyond simply specifying added ingredients. It is also imperative that any impurities are also declared given some of these have the potential to trigger adverse reactions. Given there is a strong move towards transparency in labelling in the food sector, itself supported by the Food Standards Agency and Department of Health, it is even more important that such transparency occurs with vaccines given they are administered systemically;
- Full disclosure of cases and potential cases of vaccine injury. Recent history of UK government communication around legal cases linked to vaccine injury caused by Pandemrix® and seasonal flu vaccines discovered during trials or post-marketing surveillance has been grossly inadequate. This inadequacy has only been revealed through multiple freedom of information requests under the Freedom of Information Act. Only a handful of cases have been made public, while many others have received Vaccine Damage Payments after establishing proof of vaccine causation but without any public communication of the cases or the nature of the injuries (see special report in Independent, 18 April). This non-disclosure does not afford the public a balanced view of the risks associated with a given vaccine, nor does it allow them to determine if their own health condition might make them more or less susceptible to adverse reactions;
- The Government must clarify eligibility and criteria for no-fault vaccine injury payments for Covid-19 vaccines. We have noted that the Government no longer considers citizens eligible for vaccine injury payments in the event of proven damage caused by a “pandemic influenza virus”. This exclusion was made only after the Government recognised from post-marketing surveillance that narcolepsy was a significant, albeit uncommon, autoimmune side effect of Pandemrix®. The Government must ensure that vaccine injury payments will be made to individuals injured by any approved Covid-19 vaccines, while also clarifying the level of proof required to establish causation and the statutory time limit for making such claims in relation to Covid-19 vaccines, prior to their administration to the public;
- The Government must clarify indemnity offered to vaccine manufacturers. In a reply made by the Department of Health to a freedom of information request (Your Ref: DE-1029593), it was stated that in relation to GlaxoSmithKline’s Pandemrix®, Baxter International’s Celvapan® and Sanofi Pasteur’s Liquid Smallpox Vaccine, “The Authority shall fully and completely indemnify the Contractor against all claims, proceedings, actions, legal suits, damages, legal costs and expenses and any other liabilities in respect of any death or personal injury arising from the Authority’s use of the Goods.” The indemnity, if applicable to Covid-19 vaccines, must be made public prior to the commercial release of vaccines because, ultimately, the financial burden of such indemnity lies with the taxpayer;
- The public must be informed of the extent of naturally-acquired immunity prior to public release of Covid-19 vaccines. In order to balance risk and utility, the public must be made aware of the extent of population herd immunity, which will necessitate carefully conducted, stratified, random sampling of the UK population and testing with a validated serological (antibody) test. We are aware that the Department of Health is evaluating such tests, and it is of paramount importance that comprehensive, periodic evaluation of population immunity is conducted to determine the persistence of such immunity. This would be greatly facilitated by quarterly testing of randomised, stratified samples of the national population and would not necessitate ‘universal’ testing of all individuals that has been correctly declared as not feasible. The public should also have ready access to validated antibody tests so that individuals can assess their own state of immunity prior to giving consent for vaccination;
- Parliament must be engaged to ensure due democratic process if the Government is planning to consider making Covid-19 vaccines mandatory. While the Public Health (Control of Disease) Act 1984 technically allows for the mandatory treatment of persons who are, or may be, infected, the decision to apply these emergency measures to Covid-19, when it has not been applied to any previous infectious disease, is a matter of great public importance. It is therefore critical that due democratic process is followed so that the will of the people can be factored into any such decision.
As Secretary of State for Health and Social Care, we are extremely aware of how hard you and your team have been working in an effort to protect the public interest during the current pandemic. However, it is crucially important that in the drive to provide one or more vaccines to enhance the population’s immunity to SARS-CoV-2, corners are not cut that expose the population to unnecessary risks, especially if these are undisclosed.
We look forward to receiving information about your Department’s approach to transparency of information and data surrounding Covid-19 vaccine trials, including post-marketing surveillance once initiated. We especially request your response to specific points set out in the ten discrete areas we have highlighted above.
We greatly look forward to hearing from you, or a member of your Departmental team, at your earliest convenience. Our respective emails are given below.
Yours sincerely,
Robert Verkerk MSc DIC PhD FACN
Executive and scientific director
Alliance for Natural Health International
www.anhinternational.org
Dr Damien Downing MBBS MSB
President
British Society for Ecological Medicine
www.bsem.org.uk